
Why is the nitrogen's lone pair part of $ s{p^2} $ orbital in pyridine but part of p orbital in pyrrole?
Answer
483.6k+ views
Hint: The hybridized $ s{p^2} $ orbital contains three orbitals and hybridized $ s{p^3} $ contains four orbitals. We can understand the reason by deep symmetry analysis and also considering the general aspects of the VSEPR theory.
Complete answer:
Pyrrole, is a five membered ring having the same no. of pi electrons as that of pyridine which is a six membered ring. Due to this there is an unusual extra fourth electron group on the Pyrrole’s Nitrogen. For the purpose of energetic stability i.e., aromaticity, Pyrrole has a $ s{p^2} $ hybridization, despite having four electron groups. The $ 2{p_y} $ orbital remains unhybridized, so it allows it to delocalize the electron density throughout the ring. For the hydrogen to be attached to the Pyrrole ring, the $ s{p^2} $ ring must align itself towards the hydrogen to overlap and form a bond. Therefore, the unhybridized $ 2{p_y} $ holds the lone pair of electrons.
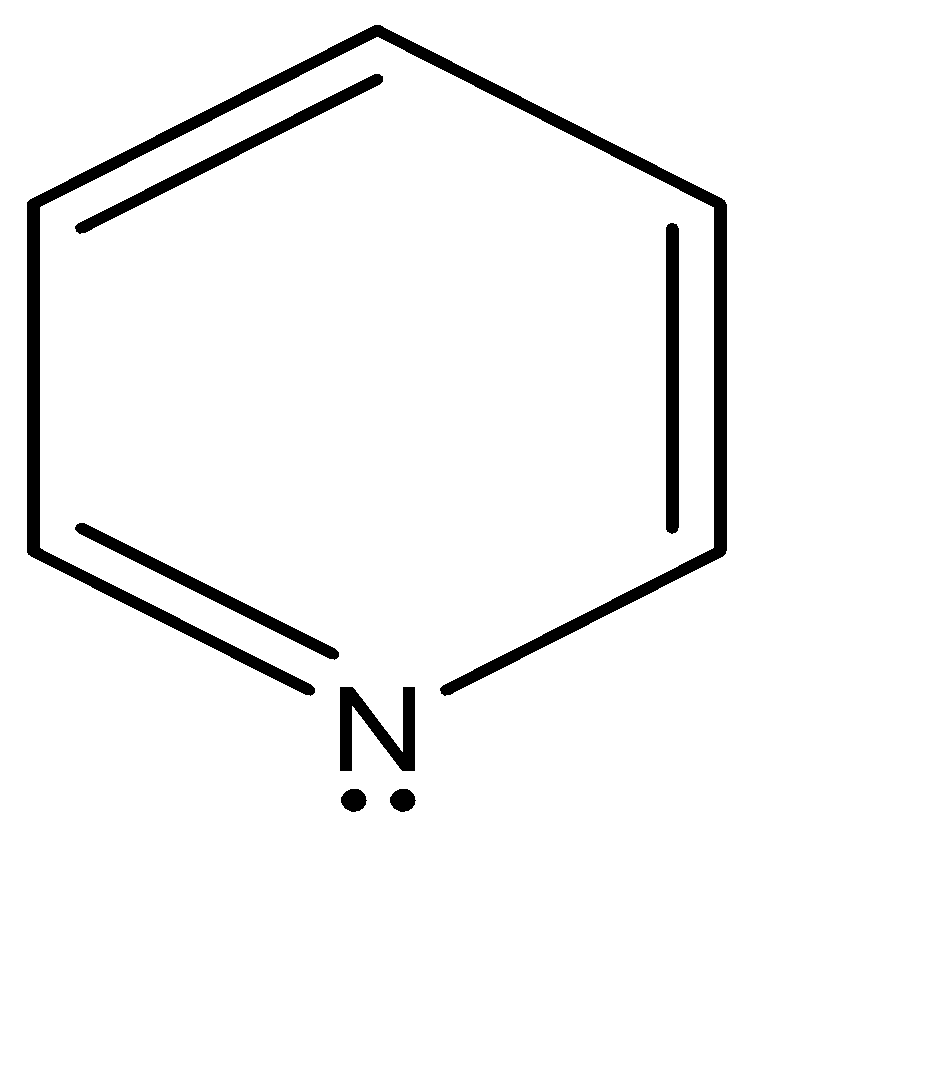
In pyridine the nitrogen has three electron groups only, therefore has the True $ s{p^2} $ hybridization. The lone pair is present on the third non bonding $ s{p^2} $ orbital. Since $ s{p^2} $ is the wrong symmetry, the electrons cannot delocalize the electron density throughout the ring. As a result, the lone pair sticks out of the ring as shown below:
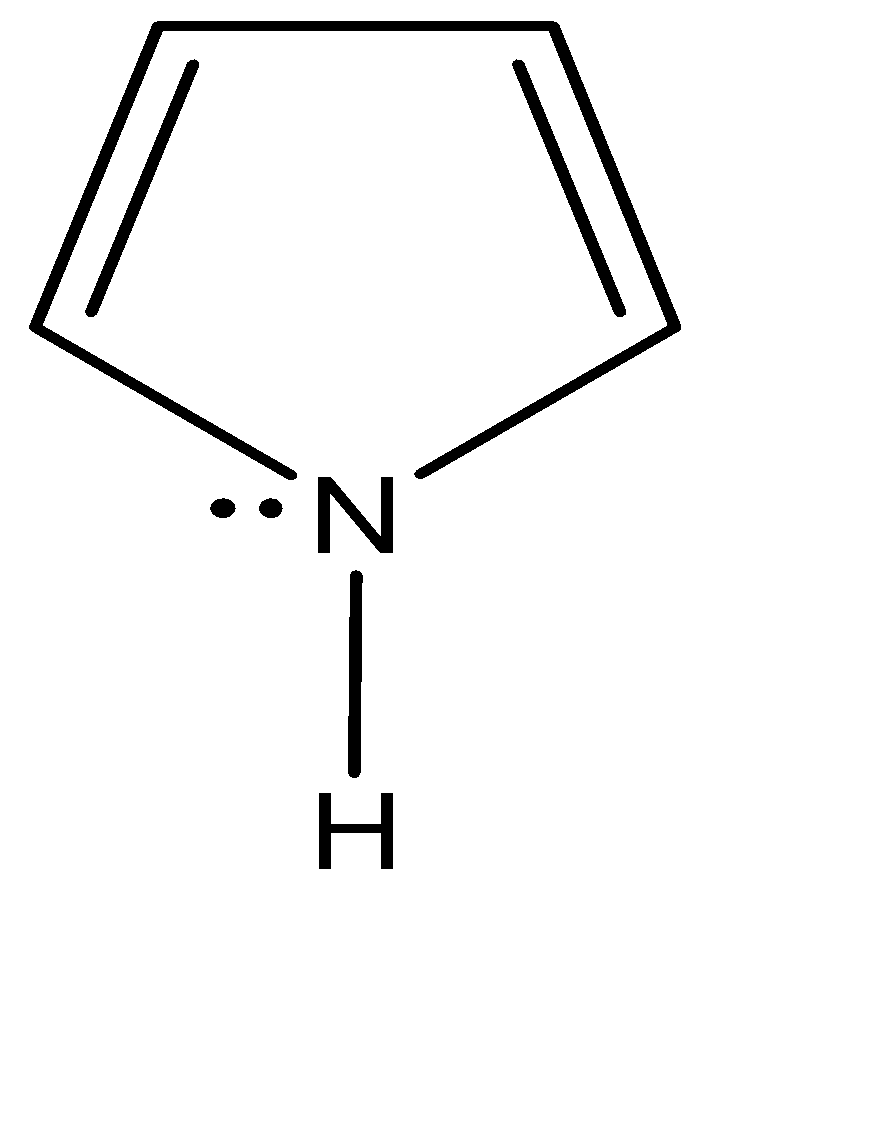
In pyrrole, the nitrogen has four electron groups but the ring constraints and the hope for aromaticity make it energetically more favourable to have the ideal $ s{p^2} $ hybridisation instead of $ s{p^3} $ . The third $ s{p^2} $ orbital is used to bond with the hydrogen. If pyrrole had $ s{p^3} $ hybridisation, it could still bond with the hydrogen but the delocalisation of the electron density would not have taken place. So, it couldn’t be aromatic, hence pyrrole has a $ s{p^2} $ hybridisation only. The fourth orbital on pyrrole holds the lone pair which helps in delocalisation of electron density due to symmetry compatibility.
Note:
The fourth orbital will not hybridize unless the aromaticity is broken. Since aromaticity is the driving factor for the stability of pyrrole, it doesn’t hybridize and holds the lone pair of electrons and promotes delocalisation.
Complete answer:
Pyrrole, is a five membered ring having the same no. of pi electrons as that of pyridine which is a six membered ring. Due to this there is an unusual extra fourth electron group on the Pyrrole’s Nitrogen. For the purpose of energetic stability i.e., aromaticity, Pyrrole has a $ s{p^2} $ hybridization, despite having four electron groups. The $ 2{p_y} $ orbital remains unhybridized, so it allows it to delocalize the electron density throughout the ring. For the hydrogen to be attached to the Pyrrole ring, the $ s{p^2} $ ring must align itself towards the hydrogen to overlap and form a bond. Therefore, the unhybridized $ 2{p_y} $ holds the lone pair of electrons.
In pyridine the nitrogen has three electron groups only, therefore has the True $ s{p^2} $ hybridization. The lone pair is present on the third non bonding $ s{p^2} $ orbital. Since $ s{p^2} $ is the wrong symmetry, the electrons cannot delocalize the electron density throughout the ring. As a result, the lone pair sticks out of the ring as shown below:

In pyrrole, the nitrogen has four electron groups but the ring constraints and the hope for aromaticity make it energetically more favourable to have the ideal $ s{p^2} $ hybridisation instead of $ s{p^3} $ . The third $ s{p^2} $ orbital is used to bond with the hydrogen. If pyrrole had $ s{p^3} $ hybridisation, it could still bond with the hydrogen but the delocalisation of the electron density would not have taken place. So, it couldn’t be aromatic, hence pyrrole has a $ s{p^2} $ hybridisation only. The fourth orbital on pyrrole holds the lone pair which helps in delocalisation of electron density due to symmetry compatibility.

Note:
The fourth orbital will not hybridize unless the aromaticity is broken. Since aromaticity is the driving factor for the stability of pyrrole, it doesn’t hybridize and holds the lone pair of electrons and promotes delocalisation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




