
Nitrogen molecule is an example of-
Answer
494.1k+ views
Hint: Atoms of nitrogen combined with another atom of nitrogen to form dinitrogen which is stable in the atmosphere. Nitrogen does not have any characteristic colour or odour. Nitrogen is a stable molecule therefore; it is considered as an inert gas at NTP.
Complete answer:
Nitrogen is first member of group $15$ element of periodic table. It is the lightest element of the entire group. Nitrogen has atomic number $7$, so according to Aufbau rule, electronic configuration of nitrogen will become $1{s^2}2{s^2}2{p^1}2{p^1}2{p^1}$. From the electronic configuration of nitrogen, we easily predict that the valence shell of nitrogen has $5$ electrons. Hence, valency of nitrogen ranges from $3$ to $5$.
When two atoms of nitrogen combine with each other there is formation of a triple bond due to interaction of three vacant orbits of $\left( p \right)$ subshell.
Chemical structure of nitrogen molecule is
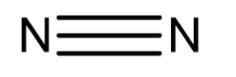
Since the bond formation is done by sharing of electrons is known as covalent bond. But as we already know that nitrogen is non-polar in nature so when it forms the bond by sharing electrons it forms a nonpolar covalent bond. Therefore, nitrogen molecules are termed non-polar molecules. Due to being non-polar in nature none of the nitrogen atoms has a tendency to shift the bonded electrons. There is generation of no partial charges over the nitrogen atom therefore molecules remain electrically neutral.
Non polar compounds have generally low solubility in water and exist mainly as gases like nitrogen gas, hydrogen gas.
$ \Rightarrow $ Nitrogen molecule is an example of nonpolar covalent compound.
Note:
Nitrogen gas is considered as the most abundant natural gas which contributes about $78\% $ of atmospheric gas. Liquid nitrogen acts as an excellent preservative to preserve natural organisms for a longer period of time. Molecules of nitrogen is non-conductor of electricity.
Complete answer:
Nitrogen is first member of group $15$ element of periodic table. It is the lightest element of the entire group. Nitrogen has atomic number $7$, so according to Aufbau rule, electronic configuration of nitrogen will become $1{s^2}2{s^2}2{p^1}2{p^1}2{p^1}$. From the electronic configuration of nitrogen, we easily predict that the valence shell of nitrogen has $5$ electrons. Hence, valency of nitrogen ranges from $3$ to $5$.
When two atoms of nitrogen combine with each other there is formation of a triple bond due to interaction of three vacant orbits of $\left( p \right)$ subshell.
Chemical structure of nitrogen molecule is

Since the bond formation is done by sharing of electrons is known as covalent bond. But as we already know that nitrogen is non-polar in nature so when it forms the bond by sharing electrons it forms a nonpolar covalent bond. Therefore, nitrogen molecules are termed non-polar molecules. Due to being non-polar in nature none of the nitrogen atoms has a tendency to shift the bonded electrons. There is generation of no partial charges over the nitrogen atom therefore molecules remain electrically neutral.
Non polar compounds have generally low solubility in water and exist mainly as gases like nitrogen gas, hydrogen gas.
$ \Rightarrow $ Nitrogen molecule is an example of nonpolar covalent compound.
Note:
Nitrogen gas is considered as the most abundant natural gas which contributes about $78\% $ of atmospheric gas. Liquid nitrogen acts as an excellent preservative to preserve natural organisms for a longer period of time. Molecules of nitrogen is non-conductor of electricity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




