
Nitrogen molecule is a nonpolar covalent molecule.
(A) True
(B) False
Answer
563.1k+ views
Hint: We know that non- polar molecules are also known as hydrophobic molecules which means water fearing and will not dissolve in water. Also, we know that covalent bond is a bond formed by the sharing of atoms. So nonpolar covalent bonds are formed when a pair of electrons are shared between two or more atoms to form a molecule where the two atoms have the same electronegativity.
Complete answer:We already know that nitrogen is a non-metal which belongs to group $15$ of the periodic table. The atomic number of nitrogen is $7$ . The nitrogen molecule is a colorless, odorless and tasteless inert gas at room temperature.
As nitrogen has an atomic number of $7$ so it will have $5$ electrons in its valence shell. Therefore, it needs only three more electrons to complete its octet. So, for this there will be mutual sharing of three pairs of electrons between two atoms of nitrogen and will thus form a triple bond.
For the better explanation we will draw and depict how ${N_2}$ will be formed.
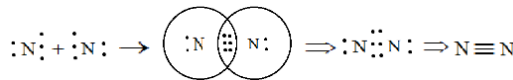
So here we see that nitrogen is forming a triple bond by sharing a total of three pairs of electrons and thus completing its octet and gaining stability. It has zero dipole moment as the two nitrogen atoms present in nitrogen molecules have the same electronegativity hence zero electronegativity difference.
So, it is true that the nitrogen molecule is a non-polar and a covalent molecule.
Hence the correct answer is option A.
Note:Nitrogen gas is an unreactive gas because the nitrogen atoms are bonded by a triple bond. So, this strong triple bond requires a high energy in order to break it. Hence the triple bond makes the nitrogen molecule a highly stable compound. Here ‘to complete its octet’ means that there should be eight electrons present in the valence shell of the atom. Helium is an exception to the octet rule as it only has two electrons in its valence shell.
Complete answer:We already know that nitrogen is a non-metal which belongs to group $15$ of the periodic table. The atomic number of nitrogen is $7$ . The nitrogen molecule is a colorless, odorless and tasteless inert gas at room temperature.
As nitrogen has an atomic number of $7$ so it will have $5$ electrons in its valence shell. Therefore, it needs only three more electrons to complete its octet. So, for this there will be mutual sharing of three pairs of electrons between two atoms of nitrogen and will thus form a triple bond.
For the better explanation we will draw and depict how ${N_2}$ will be formed.

So here we see that nitrogen is forming a triple bond by sharing a total of three pairs of electrons and thus completing its octet and gaining stability. It has zero dipole moment as the two nitrogen atoms present in nitrogen molecules have the same electronegativity hence zero electronegativity difference.
So, it is true that the nitrogen molecule is a non-polar and a covalent molecule.
Hence the correct answer is option A.
Note:Nitrogen gas is an unreactive gas because the nitrogen atoms are bonded by a triple bond. So, this strong triple bond requires a high energy in order to break it. Hence the triple bond makes the nitrogen molecule a highly stable compound. Here ‘to complete its octet’ means that there should be eight electrons present in the valence shell of the atom. Helium is an exception to the octet rule as it only has two electrons in its valence shell.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




