
Nitrogen exists as a diatomic molecule and phosphorus as ${{\text{P}}_4}$. why?
Answer
577.2k+ views
Hint:The nitrogen is of small size and phosphorus is of large size. The effective nuclear charge of nitrogen is high and the effective nuclear charge of phosphorus is less. The smaller-sized atoms can form multiple bonds whereas the large molecule cannot due to repulsion between the bonded atoms.
Complete answer:
Nitrogen and phosphorus both are the elements of the same group. Hence both have similar valence shell electronic configuration. Due to similar electronic configuration, they have many similar chemical properties.Nitrogen and phosphorus also have some differences also such as size difference, effective nuclear charge difference, etc.
As we move down in a group, the size of the metal increases. The size increases because down in a group electrons get added to the next principal quantum number so, the distance between outermost shell electrons and nucleus increases so, due to this effective nuclear charge decreases so, the size increases.
So, phosphorus is larger than nitrogen. Nitrogen has a higher effective nuclear charge than phosphorus.
Due to high effective nuclear charge and small size, the nitrogen is able to form as many bonds as it wants to complete its octant with another nitrogen atom. Valence electrons of the nitrogen are five so it requires three electrons to complete its octant so it forms a triple bond with another nitrogen atom.
The high effective nuclear charge helps to bind so much electron density in small volume in between the two nitrogen atoms. The small size makes possible the closest approach of another nitrogen atom to stabilize the diatomic nitrogen molecule so nitrogen exists as a diatomic molecule.
Due to a less effective nuclear charge and large size, the phosphorus is not able to form as many bonds as it wants to complete its octant with one another phosphorus atom. If phosphorus forms a triple bond with another phosphorus atom then due to big size the repulsion increases so much that it destabilizes the diatomic phosphorus molecule. So, it exists as${{\text{P}}_4}$ to complete its octet and minimize the repulsion.
The structure of nitrogen and ${{\text{P}}_4}$is as follows:
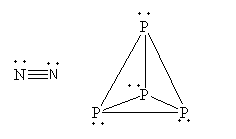
Note:Single bonds are less stable than a triple bond. So, the triple bonded nitrogen atoms are in more close proximity than the single-bonded four phosphorus atoms. The benefit to existing in the ${{\text{P}}_4}$ form is the octet of the phosphorus gets completed. Due to single bond atoms remains as far as possible to minimize the repulsion.
Complete answer:
Nitrogen and phosphorus both are the elements of the same group. Hence both have similar valence shell electronic configuration. Due to similar electronic configuration, they have many similar chemical properties.Nitrogen and phosphorus also have some differences also such as size difference, effective nuclear charge difference, etc.
As we move down in a group, the size of the metal increases. The size increases because down in a group electrons get added to the next principal quantum number so, the distance between outermost shell electrons and nucleus increases so, due to this effective nuclear charge decreases so, the size increases.
So, phosphorus is larger than nitrogen. Nitrogen has a higher effective nuclear charge than phosphorus.
Due to high effective nuclear charge and small size, the nitrogen is able to form as many bonds as it wants to complete its octant with another nitrogen atom. Valence electrons of the nitrogen are five so it requires three electrons to complete its octant so it forms a triple bond with another nitrogen atom.
The high effective nuclear charge helps to bind so much electron density in small volume in between the two nitrogen atoms. The small size makes possible the closest approach of another nitrogen atom to stabilize the diatomic nitrogen molecule so nitrogen exists as a diatomic molecule.
Due to a less effective nuclear charge and large size, the phosphorus is not able to form as many bonds as it wants to complete its octant with one another phosphorus atom. If phosphorus forms a triple bond with another phosphorus atom then due to big size the repulsion increases so much that it destabilizes the diatomic phosphorus molecule. So, it exists as${{\text{P}}_4}$ to complete its octet and minimize the repulsion.
The structure of nitrogen and ${{\text{P}}_4}$is as follows:

Note:Single bonds are less stable than a triple bond. So, the triple bonded nitrogen atoms are in more close proximity than the single-bonded four phosphorus atoms. The benefit to existing in the ${{\text{P}}_4}$ form is the octet of the phosphorus gets completed. Due to single bond atoms remains as far as possible to minimize the repulsion.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




