
Nitrogen dioxide is a covalent compound. How many oxygen atoms are in nitrogen dioxide molecules?
Answer
557.7k+ views
Hint:In covalent compounds, the atoms are bonded with a covalent bond. The molecular formula of nitrogen dioxide is $N{O_2}$. The center atom nitrogen is bonded to one oxygen from one side and other oxygen from the other side.
Complete step by step answer:
The covalent compounds are those chemical compounds where the atoms present in the chemical compound are bonded by covalent bonds.
The covalent bonds are formed by the mutual sharing of electrons by the atom to form the chemical compound.
The nitrogen dioxide is a covalent compound where one nitrogen is the central atom which is bonded to two oxygen atoms, where one oxygen atom is bonded by a single bond and other oxygen atom by a double bond. The atomic weight of nitrogen is 7. The electronic configuration of nitrogen is $[He]2{s^2}2{p^3}$, the valence electrons in nitrogen is 5. The atomic weight of oxygen is 8. The electronic configuration of oxygen is $[He]2{s^2}2{p^4}$, the valence electron in oxygen is 6. Total valence electrons present in nitrogen dioxide molecules is 17 (two oxygen atoms present).
6 electrons take part in bonding, 5 electron pairs are present on the oxygen atom and one electron present in the nitrogen.
The structure of nitrogen dioxide is shown below.
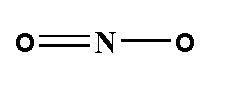
Total two oxygen atoms are present nitrogen dioxide.
Note:
In nitrogen dioxide, the double bond is formed from one sigma bond and one pi bond and the single bond is formed from one sigma bond.
Complete step by step answer:
The covalent compounds are those chemical compounds where the atoms present in the chemical compound are bonded by covalent bonds.
The covalent bonds are formed by the mutual sharing of electrons by the atom to form the chemical compound.
The nitrogen dioxide is a covalent compound where one nitrogen is the central atom which is bonded to two oxygen atoms, where one oxygen atom is bonded by a single bond and other oxygen atom by a double bond. The atomic weight of nitrogen is 7. The electronic configuration of nitrogen is $[He]2{s^2}2{p^3}$, the valence electrons in nitrogen is 5. The atomic weight of oxygen is 8. The electronic configuration of oxygen is $[He]2{s^2}2{p^4}$, the valence electron in oxygen is 6. Total valence electrons present in nitrogen dioxide molecules is 17 (two oxygen atoms present).
6 electrons take part in bonding, 5 electron pairs are present on the oxygen atom and one electron present in the nitrogen.
The structure of nitrogen dioxide is shown below.
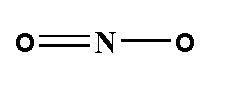
Total two oxygen atoms are present nitrogen dioxide.
Note:
In nitrogen dioxide, the double bond is formed from one sigma bond and one pi bond and the single bond is formed from one sigma bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




