
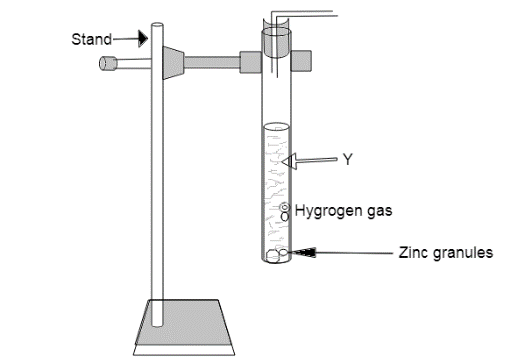
Nikita and Tarun arranged the given set up of the experiment separately. Both have performed the experiment with 2 different solutions (Y). If in case of Nikita solution (Y) is dilute Hydrochloric acid and in case of Tarun solution (Y) is sodium Hydroxide the compounds formed are -- and ----- respectively.
A. $N{a_2}Zn{O_2}$ and $ZnC{l_2}$
B. $ZnC{l_2}$ and $N{a_2}Zn{O_2}$
C. $ZnS{O_4}$ and $N{a_2}Zn{O_2}$
D. $ZnC{l_2}$ and $ZnO$

Answer
505.8k+ views
Hint: Hydrogen gas with molecular formula ${H_2}$ is a diatomic gas. At standard temperature and pressure i.e. at STP hydrogen gas is a colourless, tasteless, odourless, non-metallic, non-toxic and highly combustible gas. Hydrogen gas can be detected using a burning splint test, that is when a splint is lit and held near a test tube containing hydrogen gas, its ignites with a squeaky pop.
Complete answer:
In a displacement reaction, an ion in a compound is replaced by an ion of another element. The arrangement of metals in the decreasing order of their reactivities is referred to as reactivity series of metals or activity series. This activity series indicates that metals above hydrogen are more reactive, thus they have the capability to replace hydrogen from its compound.
In the given apparatus it is seen that zinc granules are reacting with the two different solutions(Y) of Nikita and Tarun which are dilute hydrochloric acid and sodium hydroxide respectively. It is also clear from the apparatus that when these two solutions(Y) react with zinc granules hydrogen gas is liberated which means hydrogen is replaced from its solution. Therefore, when solution(Y) of Nikita i.e. dilute hydrochloric acid reacts with zinc granules, displacement reaction takes place i.e. zinc being more reactive than hydrogen displaces it from the HCl solution to form zinc chloride.
$Z{n_{(s)}} + HC{l_{(aq)}} \to ZnC{l_{2(aq)}} + {H_{2(g)}}$
Similarly, when solution(Y) of Tarun i.e. sodium hydroxide reacts with zinc granules to form sodium zincate.
$2NaO{H_{(aq)}} + Z{n_{(s)}} \to N{a_2}Zn{O_{(aq)}} + {H_{2(g)}}$
Therefore the correct answer is option B.
Note:
Reactivity series is used to predict whether a metal can displace another element in a single displacement reaction. Moreover, the metals at the top of the reactivity series are powerful reducing agents i.e. they themselves undergo oxidation by losing electrons.
Complete answer:
In a displacement reaction, an ion in a compound is replaced by an ion of another element. The arrangement of metals in the decreasing order of their reactivities is referred to as reactivity series of metals or activity series. This activity series indicates that metals above hydrogen are more reactive, thus they have the capability to replace hydrogen from its compound.
In the given apparatus it is seen that zinc granules are reacting with the two different solutions(Y) of Nikita and Tarun which are dilute hydrochloric acid and sodium hydroxide respectively. It is also clear from the apparatus that when these two solutions(Y) react with zinc granules hydrogen gas is liberated which means hydrogen is replaced from its solution. Therefore, when solution(Y) of Nikita i.e. dilute hydrochloric acid reacts with zinc granules, displacement reaction takes place i.e. zinc being more reactive than hydrogen displaces it from the HCl solution to form zinc chloride.
$Z{n_{(s)}} + HC{l_{(aq)}} \to ZnC{l_{2(aq)}} + {H_{2(g)}}$
Similarly, when solution(Y) of Tarun i.e. sodium hydroxide reacts with zinc granules to form sodium zincate.
$2NaO{H_{(aq)}} + Z{n_{(s)}} \to N{a_2}Zn{O_{(aq)}} + {H_{2(g)}}$
Therefore the correct answer is option B.
Note:
Reactivity series is used to predict whether a metal can displace another element in a single displacement reaction. Moreover, the metals at the top of the reactivity series are powerful reducing agents i.e. they themselves undergo oxidation by losing electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




