
Nickel atoms can lose two electrons to form $Ni^{2+}$ ions. The atomic number of nickel is $\;28$ . From which orbital it can lose two electrons?
A) $Ni={{[Ar]}^{18}}3{{d}^{8}}4{{s}^{2}}$ . To form $Ni^{2+}$ ion, it will lose electrons from $4\;s$
B) $Ni={{[Ar]}^{18}}3{{d}^{8}}4{{s}^{2}}$ . To form $Ni^{2+}$ ion, it will lose electrons from $3\;d$
C) Both from $3\;d$ and $4\;s$
D) None of the above
Answer
527.4k+ views
Hint: Nickel element belongs to the fourth period and tenth group of the periodic table. It belongs to the $d$ -block elements also known as transition elements. The last electron entering will enter the $d$ -orbital. To become a positive ion, the electrons that experience least attraction force from the nucleus and do not increase instability on removal, will be eliminated.
Complete answer:
Nickel is the eighth element in the first period of $d$ -block. Hence, it contains eight electrons in the $d$ -orbital. The atomic number of Nickel $Ni\;$ is $28\;$ . Hence, the electronic configuration is given as
$Ni=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{8}}$
Considering the configuration of the previous nearest inert gas, the electronic configuration with inert gas core can be written as
$Ni=\left[ Ar \right]4{{s}^{2}}3{{d}^{8}}$
Now, we can write the electron configuration of these last two orbitals with the spin of electrons as shown below;
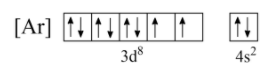
Now, in nickel, the last electron enters the $d$ -orbital. Hence, as per the general condition, while eliminating electrons, the electrons will be removed from the $d$ -orbital.
As per the given condition, let us assume Nickel removes two electrons from the $d$ -orbital as shown below;
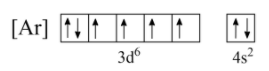
Now, from the above configuration, if electrons are eliminated from the $d$ -orbital, it will increase the number of unpaired electrons, and as unpaired electrons are not stable, the stability of the ion $Ni^{2+}$ decreases. Hence, electrons will not be removed from the $\;3d$ orbital.
Now, for the $4s\;$ orbital, even though its energy level is lower than $3d\;$ it is located farthest from the nucleus, and hence experiences least attraction from the nucleus. Hence, we can eliminate both electrons of $4s\;$ orbital as shown below;
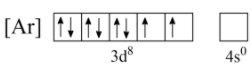
This configuration does not decrease the number of unpaired electrons, but it also does not increase it. Hence, this configuration is comparatively more stable.
Hence, Nickel will remove two electrons from $4s\;$ orbital and become $Ni^{2+}$ ion.
Hence, the correct answer is Option $(A)$
Note: In normal state, all five $d$ -orbitals of nickel will be degenerated i.e. at the same energy level. Hence, removing two electrons increases the number of unpaired electrons, which decreases stability. Hence, electrons will be removed from $s$ -orbital. However, in the presence of ligands, the five orbitals do not remain degenerated, but get split into two levels. The lower energy level $t_{2g}$ which contains three orbitals, completely filled in nickel and the higher energy level $e_g$ which contains two orbitals, both with unpaired electrons in nickel. Hence, two remove paramagnetic effects of nickel, two electrons of $s$ -orbital move to the $d$ -orbital and increase stability.
Complete answer:
Nickel is the eighth element in the first period of $d$ -block. Hence, it contains eight electrons in the $d$ -orbital. The atomic number of Nickel $Ni\;$ is $28\;$ . Hence, the electronic configuration is given as
$Ni=1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{8}}$
Considering the configuration of the previous nearest inert gas, the electronic configuration with inert gas core can be written as
$Ni=\left[ Ar \right]4{{s}^{2}}3{{d}^{8}}$
Now, we can write the electron configuration of these last two orbitals with the spin of electrons as shown below;

Now, in nickel, the last electron enters the $d$ -orbital. Hence, as per the general condition, while eliminating electrons, the electrons will be removed from the $d$ -orbital.
As per the given condition, let us assume Nickel removes two electrons from the $d$ -orbital as shown below;

Now, from the above configuration, if electrons are eliminated from the $d$ -orbital, it will increase the number of unpaired electrons, and as unpaired electrons are not stable, the stability of the ion $Ni^{2+}$ decreases. Hence, electrons will not be removed from the $\;3d$ orbital.
Now, for the $4s\;$ orbital, even though its energy level is lower than $3d\;$ it is located farthest from the nucleus, and hence experiences least attraction from the nucleus. Hence, we can eliminate both electrons of $4s\;$ orbital as shown below;

This configuration does not decrease the number of unpaired electrons, but it also does not increase it. Hence, this configuration is comparatively more stable.
Hence, Nickel will remove two electrons from $4s\;$ orbital and become $Ni^{2+}$ ion.
Hence, the correct answer is Option $(A)$
Note: In normal state, all five $d$ -orbitals of nickel will be degenerated i.e. at the same energy level. Hence, removing two electrons increases the number of unpaired electrons, which decreases stability. Hence, electrons will be removed from $s$ -orbital. However, in the presence of ligands, the five orbitals do not remain degenerated, but get split into two levels. The lower energy level $t_{2g}$ which contains three orbitals, completely filled in nickel and the higher energy level $e_g$ which contains two orbitals, both with unpaired electrons in nickel. Hence, two remove paramagnetic effects of nickel, two electrons of $s$ -orbital move to the $d$ -orbital and increase stability.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




