
Name the simplest hydrocarbon.
Answer
611.7k+ views
Hint: Simplest hydrocarbon belongs to the saturated class of hydrocarbons having a single bond in their structure.
Complete answer:
In organic chemistry a hydrocarbon is a chemical compound which consists of carbon atoms and hydrogen atoms. These hydrocarbons occur naturally and they are the basis of natural gas, coal, crude oil and other energy resources.
Types of hydrocarbons:
Hydrocarbons are classified as saturated hydrocarbons, unsaturated hydrocarbon and aromatic hydrocarbons.
Saturated hydrocarbons are the simplest form of hydrocarbons they consists of single bond and are saturated with hydrogens, the formula for acyclic hydrocarbon(open chain compound) i.e alkanes is \[{{\text{C}}_n}{{\text{H}}_{2n + 2}}\] where ${\text{n}}$ is the number of carbon atom.
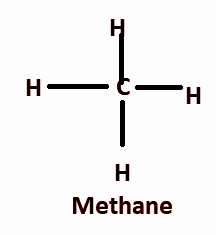
And methane is the simplest hydrocarbon which contains one carbon bonded to four hydrogen atoms.
Unsaturated hydrocarbons consists of one or more double bonds and triple bonds between the carbon atoms, those having double bonds they are called as alkenes and they have the formula \[{{\text{C}}_n}{{\text{H}}_{2n}}\] and those having triple bonds they are called as alkynes and they have the formula \[{{\text{C}}_n}{{\text{H}}_{2n - 2}}\].
Third one are aromatic hydrocarbons, they are also known as arenes(having a sigma bond and delocalized pi electrons between carbon atoms forming a circle) and they have at least one aromatic ring or we can say cyclic ring in their structure.
Note: Methane is the simplest alkane and alkanes have the formula \[{{\text{C}}_n}{{\text{H}}_{2n + 2}}\] where ${\text{n}}$ is the number of carbon atom, so methane contains one carbon atom and the number of hydrogen atoms are four i.e. \[{{\text{C}}_1}{{\text{H}}_{2 \times 1 + 2}} = {\text{C}}{{\text{H}}_4}\].
Complete answer:
In organic chemistry a hydrocarbon is a chemical compound which consists of carbon atoms and hydrogen atoms. These hydrocarbons occur naturally and they are the basis of natural gas, coal, crude oil and other energy resources.
Types of hydrocarbons:
Hydrocarbons are classified as saturated hydrocarbons, unsaturated hydrocarbon and aromatic hydrocarbons.
Saturated hydrocarbons are the simplest form of hydrocarbons they consists of single bond and are saturated with hydrogens, the formula for acyclic hydrocarbon(open chain compound) i.e alkanes is \[{{\text{C}}_n}{{\text{H}}_{2n + 2}}\] where ${\text{n}}$ is the number of carbon atom.
And methane is the simplest hydrocarbon which contains one carbon bonded to four hydrogen atoms.

Unsaturated hydrocarbons consists of one or more double bonds and triple bonds between the carbon atoms, those having double bonds they are called as alkenes and they have the formula \[{{\text{C}}_n}{{\text{H}}_{2n}}\] and those having triple bonds they are called as alkynes and they have the formula \[{{\text{C}}_n}{{\text{H}}_{2n - 2}}\].
Third one are aromatic hydrocarbons, they are also known as arenes(having a sigma bond and delocalized pi electrons between carbon atoms forming a circle) and they have at least one aromatic ring or we can say cyclic ring in their structure.
Note: Methane is the simplest alkane and alkanes have the formula \[{{\text{C}}_n}{{\text{H}}_{2n + 2}}\] where ${\text{n}}$ is the number of carbon atom, so methane contains one carbon atom and the number of hydrogen atoms are four i.e. \[{{\text{C}}_1}{{\text{H}}_{2 \times 1 + 2}} = {\text{C}}{{\text{H}}_4}\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




