
Name the scientist who first formulated the atomic structure.
Answer
587.4k+ views
Hint: The atomic structure consists of Atomic particles. Atoms consist of three basic particles, protons, electrons and neutrons. The nucleus (center) of an atom contains the protons (positively charged) and the neutrons (no charge). The outermost regions of the atom are called electron shells and they contain the electrons.
Complete step by step answer:
-The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons, electrons and neutrons.
-But, the atoms of different elements have different atomic structures because they contain different numbers of protons and electrons.
-The first scientific theory of atomic structure was proposed by John Dalton in1800s. The atomic structure is as shown-
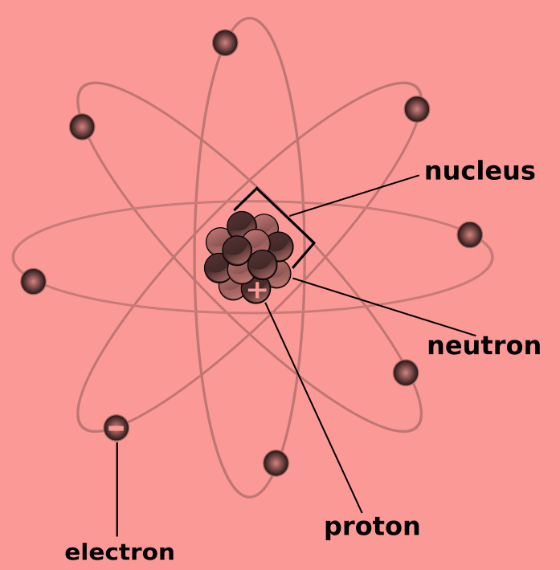
John Dalton suggested that all matter is made up of atoms, which were invisible and indestructible. He also stated that all the atoms of an element were exactly the same, but the atoms of different elements differ in size and mass.
Note:
There were few postulates of this theory:
-Every matter is made up of atoms.
-Atoms are invisible.
-Specific elements have only one type of atom in them.
-Each atom has its own constant mass that varies from element to element.
-Atoms undergo rearrangement during a chemical reaction.
Complete step by step answer:
-The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons, electrons and neutrons.
-But, the atoms of different elements have different atomic structures because they contain different numbers of protons and electrons.
-The first scientific theory of atomic structure was proposed by John Dalton in1800s. The atomic structure is as shown-

John Dalton suggested that all matter is made up of atoms, which were invisible and indestructible. He also stated that all the atoms of an element were exactly the same, but the atoms of different elements differ in size and mass.
Note:
There were few postulates of this theory:
-Every matter is made up of atoms.
-Atoms are invisible.
-Specific elements have only one type of atom in them.
-Each atom has its own constant mass that varies from element to element.
-Atoms undergo rearrangement during a chemical reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




