
Name the kind of particles present in carbonic acid.
Answer
556.8k+ views
Hint:Particles here are ions in other words which combine to form molecules. Take the easiest example of water if we talk about it, it is formed from two ions one is hydrogen ion and another is hydroxyl ion. When these two ions combine in electric discharge they form water. Similarly try to understand firstly the formula for carbonic acid and then find out the ions.
Complete step-by-step answer:Carbonic acid is a dibasic acid, it means it contains two basic hydrogen which it gives up easily. If we write the molecular formula of carbonic acid, it will be like this ${H_2}C{O_3}$ . There is oxygen joined with carbon with a double bond and other valances are satisfied by the OH group connected with single bonds. Let’s see the picture, there is no lone pair here all valences of carbon are satisfied.
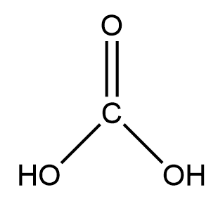
We can find out the particles from it is formed or by decomposing it. When ${H_2}C{O_3}$ get decompose, let’s say take one hydrogen from its structure you will get
${H_2}C{O_3} \to \,{H^ + }\, + \,HC{O_3}^ - $ Further if it get dissociates we will get one more hydrogen ion ${H^ + }\,$ and carbonate ion $CO_3^{2 - }$ .
$HC{O_3}^ - \to \,{H^ + }\, + \,CO_3^{2 - }$
Now the two equations are just before us, we get an idea that the particles from carbonic acid are formed are hydrogen ion and carbonate ion.
Note:Keep in mind that carbonic acid is formed directly by hydrogen ions and carbonate ions. When two moles of hydrogen ions reacts with one mole of carbonate ion then one mole of carbonic acid is formed. When you hear about particles you must think of mole concept and all but here the particles are just atoms or ions from which a compound is formed.
Complete step-by-step answer:Carbonic acid is a dibasic acid, it means it contains two basic hydrogen which it gives up easily. If we write the molecular formula of carbonic acid, it will be like this ${H_2}C{O_3}$ . There is oxygen joined with carbon with a double bond and other valances are satisfied by the OH group connected with single bonds. Let’s see the picture, there is no lone pair here all valences of carbon are satisfied.

We can find out the particles from it is formed or by decomposing it. When ${H_2}C{O_3}$ get decompose, let’s say take one hydrogen from its structure you will get
${H_2}C{O_3} \to \,{H^ + }\, + \,HC{O_3}^ - $ Further if it get dissociates we will get one more hydrogen ion ${H^ + }\,$ and carbonate ion $CO_3^{2 - }$ .
$HC{O_3}^ - \to \,{H^ + }\, + \,CO_3^{2 - }$
Now the two equations are just before us, we get an idea that the particles from carbonic acid are formed are hydrogen ion and carbonate ion.
Note:Keep in mind that carbonic acid is formed directly by hydrogen ions and carbonate ions. When two moles of hydrogen ions reacts with one mole of carbonate ion then one mole of carbonic acid is formed. When you hear about particles you must think of mole concept and all but here the particles are just atoms or ions from which a compound is formed.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




