
Name the IUPAC name of neopentane.
A. 2-methylbutane
B. 2, 2-dimethylpropane
C. 2-methylpropane
D. 2, 2-dimethylbutane
Answer
558.6k+ views
Hint:First of all we should be aware of IUPAC. The method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC) is known as the IUPAC nomenclature of a compound.
Complete step-by-step answer:Rules of writing IUPAC name of a compound.
A. For the identification of parent chain
1.It should consist of a maximum number of substituents or branches as prefixes.
2.It should consist of the maximum number of substituents of the suffix functional group.
3.Must have the maximum length.
4.Must contains the maximum number of multiple bonds.
5.Should have maximum number of single bonds.
B. The highest order of presence is required for the identification of functional group.
C. Numbering of the carbon atom should be done in the parent chain, from the end which gives the substituents as the lowest numbers.
D. In a condition, where the same substituent occurs more than once then the substituent group is indicated by a prefix (di, tri, tetra, etc.)
E. In the case of two or more different substituents they should be written in alphabetical order
F. A cyclic or ring hydrocarbon is written by the prefix cyclo- before their base name.
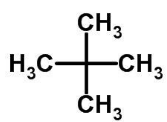
The correct option is B, which IUPAC name of neopentane is 2, 2-dimethylpropane.
Note:The main aim of IUPAC nomenclature is to provide each structure a unique and unambiguous name, and to give each name a unique and unambiguous structure. It creates a standardized way to name chemical compounds.
Complete step-by-step answer:Rules of writing IUPAC name of a compound.
A. For the identification of parent chain
1.It should consist of a maximum number of substituents or branches as prefixes.
2.It should consist of the maximum number of substituents of the suffix functional group.
3.Must have the maximum length.
4.Must contains the maximum number of multiple bonds.
5.Should have maximum number of single bonds.
B. The highest order of presence is required for the identification of functional group.
C. Numbering of the carbon atom should be done in the parent chain, from the end which gives the substituents as the lowest numbers.
D. In a condition, where the same substituent occurs more than once then the substituent group is indicated by a prefix (di, tri, tetra, etc.)
E. In the case of two or more different substituents they should be written in alphabetical order
F. A cyclic or ring hydrocarbon is written by the prefix cyclo- before their base name.

The correct option is B, which IUPAC name of neopentane is 2, 2-dimethylpropane.
Note:The main aim of IUPAC nomenclature is to provide each structure a unique and unambiguous name, and to give each name a unique and unambiguous structure. It creates a standardized way to name chemical compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




