
Name the hardest non metal -
Answer
496.8k+ views
Hint: A nonmetal is a chemical element that, in its most stable state, is a gas, liquid, or fragile solid, and which generally acquires or shares electrons in chemical processes. Nonmetals often have a drab look, low melting temperatures, boiling boils, and densities, and are poor heat and electrical conductors. Nonmetals have greater ionization energies, electron affinity, and electronegativity than metals, and their oxides are acidic. A variety of additional features are shared by most or some nonmetals; a few exhibit anomalous traits.
Complete answer:
Carbon allotropes such as diamond and graphite are well-known. The carbon atoms in a diamond are organized tetrahedrally. Each carbon atom has four other carbon atoms linked to it. It's a three-dimensional structure with a strong, rigid structure that results in an endless network of atoms. The hardness of a diamond is due to this.
The structurally diverse forms of the same element are known as allotropes. They both have the same element's atoms, but the configurations in which these atoms are organized differ significantly. As a result, several physical forms of the same element emerge. Even though they are made up of the same element's molecules, allotropes have different physical and chemical characteristics.
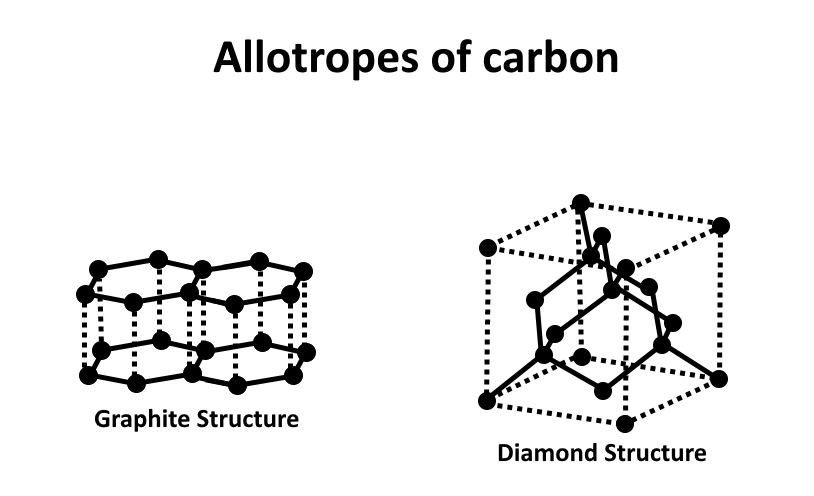
A diamond's carbon atoms are covalently linked to four other carbon atoms to form a tetrahedral structure. These tetrahedrons combine to form a three-dimensional carbon ring network. Diamond is the hardest allotrope of carbon and the hardest mineral due to its stable network of covalent connections and hexagonal rings. Each carbon atom in graphite is connected to three other carbon atoms via a covalent connection. Layers of carbon atoms with a hexagonal arrangement of atoms develop.
Because of its valency, carbon is known to produce a variety of allotropes. There are eight different types of carbon allotropes, the most prevalent of which being diamond and graphite.
Hence Diamond is the strongest non-metal.
Note:
Diamond is a kind of carbon that has its atoms organized in a diamond cubic crystal structure. Another solid form of carbon known as graphite is the chemically stable form of carbon at normal temperature and pressure, although diamond nearly never transforms to it. Diamond has the highest hardness and thermal conductivity of any natural substance, characteristics that make it ideal for cutting and polishing equipment in industry. They're also why diamond anvil cells may expose materials to pressures encountered deep down.
Complete answer:
Carbon allotropes such as diamond and graphite are well-known. The carbon atoms in a diamond are organized tetrahedrally. Each carbon atom has four other carbon atoms linked to it. It's a three-dimensional structure with a strong, rigid structure that results in an endless network of atoms. The hardness of a diamond is due to this.
The structurally diverse forms of the same element are known as allotropes. They both have the same element's atoms, but the configurations in which these atoms are organized differ significantly. As a result, several physical forms of the same element emerge. Even though they are made up of the same element's molecules, allotropes have different physical and chemical characteristics.

A diamond's carbon atoms are covalently linked to four other carbon atoms to form a tetrahedral structure. These tetrahedrons combine to form a three-dimensional carbon ring network. Diamond is the hardest allotrope of carbon and the hardest mineral due to its stable network of covalent connections and hexagonal rings. Each carbon atom in graphite is connected to three other carbon atoms via a covalent connection. Layers of carbon atoms with a hexagonal arrangement of atoms develop.
Because of its valency, carbon is known to produce a variety of allotropes. There are eight different types of carbon allotropes, the most prevalent of which being diamond and graphite.
Hence Diamond is the strongest non-metal.
Note:
Diamond is a kind of carbon that has its atoms organized in a diamond cubic crystal structure. Another solid form of carbon known as graphite is the chemically stable form of carbon at normal temperature and pressure, although diamond nearly never transforms to it. Diamond has the highest hardness and thermal conductivity of any natural substance, characteristics that make it ideal for cutting and polishing equipment in industry. They're also why diamond anvil cells may expose materials to pressures encountered deep down.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




