
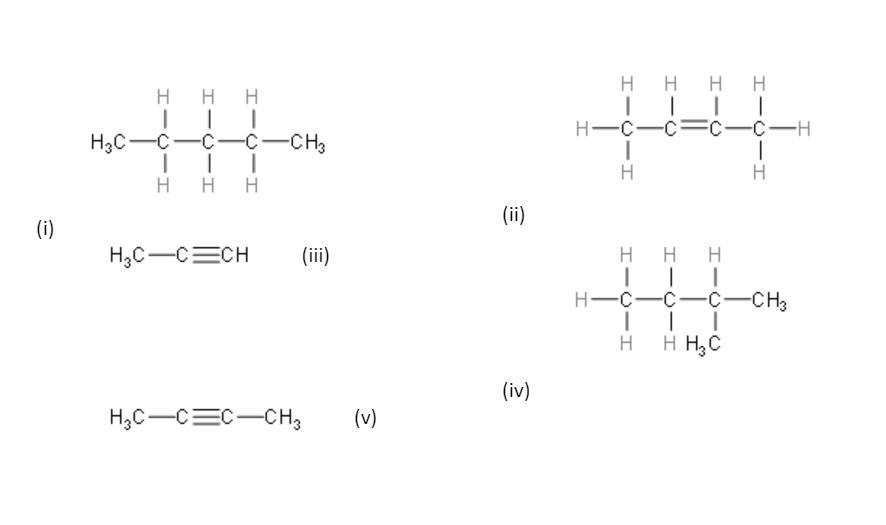
Name the following compounds.

Answer
597k+ views
Hint: First of all you have to find out the longest carbon chain. Then identify the groups attached to this chain. Now, number the chain consecutively, starting at the end nearest a substituent group by an appropriate number and name.
Complete step by step answer:
For the first compound, the longest carbon chain is pentane. There are no other substituent groups attached to this chain. So, the name of the compound is n-pentane.
For the second compound, the longest carbon chain is butane. There is a double bond in the second position. So, the name is but-2-ene.
For the third compound, the longest carbon chain is propane. There is a triple bond in the first carbon. So, the name of the compound is prop-1-yne or propyne.
For the fourth compound, the longest carbon chain is butane. There is a methyl group attached to the second carbon of the chain. So, the name is 2-methyl-butane.
For the fifth compound, the longest carbon chain is butane. There are no substituent groups attached to the chain. But there is a triple bond in the second carbon. So, the name is but-2-yne.
So, compound (i) is n-pentane.
Compound (ii) is but-2-ene.
Compound (iii) is prop-1-yne.
Compound (iv) is 2-methyl-butane.
Compound (v) is but-2-yne.
Note: When there are two or more substituent groups, you should number the chain by considering that the highest priority group like acid groups should have the lowest number. Also if two same substituent groups are attached at two different positions, numbering should be done in such a way that the sum of the number of positions has a lower number.

Complete step by step answer:
For the first compound, the longest carbon chain is pentane. There are no other substituent groups attached to this chain. So, the name of the compound is n-pentane.
For the second compound, the longest carbon chain is butane. There is a double bond in the second position. So, the name is but-2-ene.
For the third compound, the longest carbon chain is propane. There is a triple bond in the first carbon. So, the name of the compound is prop-1-yne or propyne.
For the fourth compound, the longest carbon chain is butane. There is a methyl group attached to the second carbon of the chain. So, the name is 2-methyl-butane.
For the fifth compound, the longest carbon chain is butane. There are no substituent groups attached to the chain. But there is a triple bond in the second carbon. So, the name is but-2-yne.
So, compound (i) is n-pentane.
Compound (ii) is but-2-ene.
Compound (iii) is prop-1-yne.
Compound (iv) is 2-methyl-butane.
Compound (v) is but-2-yne.
Note: When there are two or more substituent groups, you should number the chain by considering that the highest priority group like acid groups should have the lowest number. Also if two same substituent groups are attached at two different positions, numbering should be done in such a way that the sum of the number of positions has a lower number.

Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




