
Name the compound with bond angles that are exactly $ 109 $ and degree $ 28 $ .
Answer
506.4k+ views
Hint: To answer this question we should have a basic idea about Bond angles. Bond angle can be defined as the angle made by two covalent bonds, these two covalent bonds are produced by the same atom. We will discuss more about the bond angle in this question to understand it better for identifying the required compound.
Complete answer:
Bond angle is a Bond property or bond parameter that provides information about the molecular geometry of the compound. Bond angle depends upon several factors which are
Number of lone pairs: If there are more lone pairs in the compound then there will be lone pair-bond pair repulsion and bond angle is reduced.
Size of central atom and surrounding atoms: If the size of atoms is larger than the electrons have more space to occupy and they face less repulsion from other electrons and gap between bond pairs increases resulting in increasing the bond angle.
Electronegativity of central atoms of surrounding atoms: If atoms are more electronegative than the electrons will be attracted more towards them resulting in decreasing the bond angle.
A compound named Carbon tetrachloride has bond angles that are exactly $ 109 $ and $ 28 $ degrees. let’s see its structure
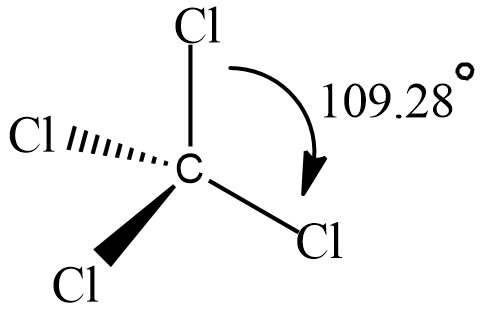
It has tetrahedral geometry and each $ C - Cl $ bond has a bond angle $ {109.28^\circ } $.
Note:
The bond angle provides us a rough idea on location of lone pairs as well as bond pairs, it also tells us about the location of the central atom and surrounding atoms in the compound so that we can determine the shape and geometry of the compound.
Complete answer:
Bond angle is a Bond property or bond parameter that provides information about the molecular geometry of the compound. Bond angle depends upon several factors which are
Number of lone pairs: If there are more lone pairs in the compound then there will be lone pair-bond pair repulsion and bond angle is reduced.
Size of central atom and surrounding atoms: If the size of atoms is larger than the electrons have more space to occupy and they face less repulsion from other electrons and gap between bond pairs increases resulting in increasing the bond angle.
Electronegativity of central atoms of surrounding atoms: If atoms are more electronegative than the electrons will be attracted more towards them resulting in decreasing the bond angle.
A compound named Carbon tetrachloride has bond angles that are exactly $ 109 $ and $ 28 $ degrees. let’s see its structure

It has tetrahedral geometry and each $ C - Cl $ bond has a bond angle $ {109.28^\circ } $.
Note:
The bond angle provides us a rough idea on location of lone pairs as well as bond pairs, it also tells us about the location of the central atom and surrounding atoms in the compound so that we can determine the shape and geometry of the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




