
What is the name of this compound NaClO ?
Answer
577.2k+ views
Hint:. NaClO is produced by the process known as the Hooker process and it is the only large-scale industrial method of NaClO production. In the process, NaClO and sodium chloride (NaCl) are formed when chlorine is passed into cold dilute sodium hydroxide (NaOH) solution.
Complete step by step answer:
Now as we know that hypochlorite is an ion composed of chlorine (Cl) and oxygen (O), and the chemical formula of hypochlorite is ClO and its structure will be
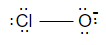
Now hypochlorite can combine with a number of different compounds to form hypochlorites which can be said as the salts of hypochlorous like bleaching powder.
And one of the compounds sodium hypochlorite is formed when sodium is combined with hypochlorite. So, the name of sodium hypochlorite must be NaClO.
And NaClO is also used in bleaching powder.
Hence, the name of the compound NaClO will be Sodium Hypochlorite.
Note: We should remember that to decompose sodium hypochlorite (NaClO) into sodium chlorite \[\left( {NaCl{O_2}} \right)\] and sodium chloride (NaCl) we had to use NaOH (Sodium Hydroxide) because NaOH is a very strong base is used. And bases are the compounds that have an OH negative ion. And it is used to neutralize the acidic effect of an acid. It is used to make common salt too.
Complete step by step answer:
Now as we know that hypochlorite is an ion composed of chlorine (Cl) and oxygen (O), and the chemical formula of hypochlorite is ClO and its structure will be
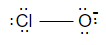
Now hypochlorite can combine with a number of different compounds to form hypochlorites which can be said as the salts of hypochlorous like bleaching powder.
And one of the compounds sodium hypochlorite is formed when sodium is combined with hypochlorite. So, the name of sodium hypochlorite must be NaClO.
And NaClO is also used in bleaching powder.
Hence, the name of the compound NaClO will be Sodium Hypochlorite.
Note: We should remember that to decompose sodium hypochlorite (NaClO) into sodium chlorite \[\left( {NaCl{O_2}} \right)\] and sodium chloride (NaCl) we had to use NaOH (Sodium Hydroxide) because NaOH is a very strong base is used. And bases are the compounds that have an OH negative ion. And it is used to neutralize the acidic effect of an acid. It is used to make common salt too.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




