
What is the name of an electrode that is joined with the negative terminal of a battery?
A. Anode
B. Cathode
C. electrolyte
D. None of these
Answer
598.8k+ views
Hint: Anode is a type of electrode which attracts negative charges and Cathode is a type of electrode which attracts positive charges. In order to have a current flow in circuit positive charges must be collected by negative terminal of battery and negative charges must be collected by a positive terminal.
Complete step-by-step answer:
Diagram:
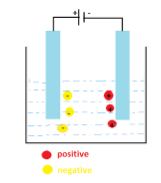
An electrode is a solid electric conductor which is used for electric conduction from one part to another. It carries electricity within itself.
There are two types of electrodes.
Anode: It attracts anions.
Cathode: It attracts cations
We know that in metals current flows due to the transport of electrons within the conductor. But in electrolytes, the current is the result of the transport of positively charged cations and negatively charged anions. When we talk about batteries, molecules contain solutions split in positive and negative ions known as cation and anions. As you can see in the figure, when an electric circuit is formed using a battery, cations shown in red colour are attracted towards the negative terminal of the battery and anions are shown in yellow colour are attracted towards the positive terminal of a battery via electrode shown in blue colour. Hence in order to have current flow in a circuit, the negative terminal of the battery must be connected to the cathode and positive terminal of a battery to the anode.
Correct option is: B - Cathode
Additional Information:
The electrode is just a medium to pass current. The reduction process takes place at the cathode and oxidation process takes place at the anode.
Note: Cathode is an electrode from where conventional current leaves and anode is an electrode from where the conventional current enters. So positive charges must accumulate at the cathode and negative charges must accumulate at the anode. In order to accumulate positive charges terminal must be negative and to accumulate negative charges terminal must be positive.
Complete step-by-step answer:
Diagram:

An electrode is a solid electric conductor which is used for electric conduction from one part to another. It carries electricity within itself.
There are two types of electrodes.
Anode: It attracts anions.
Cathode: It attracts cations
We know that in metals current flows due to the transport of electrons within the conductor. But in electrolytes, the current is the result of the transport of positively charged cations and negatively charged anions. When we talk about batteries, molecules contain solutions split in positive and negative ions known as cation and anions. As you can see in the figure, when an electric circuit is formed using a battery, cations shown in red colour are attracted towards the negative terminal of the battery and anions are shown in yellow colour are attracted towards the positive terminal of a battery via electrode shown in blue colour. Hence in order to have current flow in a circuit, the negative terminal of the battery must be connected to the cathode and positive terminal of a battery to the anode.
Correct option is: B - Cathode
Additional Information:
The electrode is just a medium to pass current. The reduction process takes place at the cathode and oxidation process takes place at the anode.
Note: Cathode is an electrode from where conventional current leaves and anode is an electrode from where the conventional current enters. So positive charges must accumulate at the cathode and negative charges must accumulate at the anode. In order to accumulate positive charges terminal must be negative and to accumulate negative charges terminal must be positive.
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




