
$ Na{H_2}P{O_4} $ and $ NaHP{O_3} $ are acid salts.
(A) True
(B) False
Answer
535.5k+ views
Hint: We know that $ Na{H_2}P{O_4} $ and $ NaHP{O_3} $ are the oxoacids (also known as oxyacid) of Phosphorous. Oxoacids are those acids that contain oxygen bonded with hydrogen and one more metal. If these oxoacids will contain more than one acidic hydrogen atom, then only the salt will be an acidic salt whereas if the compound will contain only one acidic hydrogen, the compound will then be called normal or neutral salts.
Complete step by step solution:
First let us see what an acid salt is:
Acid salt: An acid is the one which forms an acidic solution when it is dissolved in water. It is formed by partial or incomplete neutralization of acid by a base. An acid salt should contain at least two replaceable hydrogen ions. Let us have a look on $ Na{H_2}P{O_4} $ and $ NaHP{O_3} $ one by one:
$ Na{H_2}P{O_4} $: $ Na{H_2}P{O_4} $ is actually formed when phosphoric acid reacts with sodium hydroxide react in the molar ratio of $ 1:1 $ as shown in the reaction below:
$ NaOH + {H_3}P{O_4} \to Na{H_2}P{O_4} + {H_2}O $
Now let’s have a look on the structure of $ Na{H_2}P{O_4} $
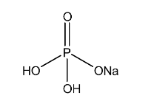
$ Na{H_2}P{O_4} $ is an acid salt because it contains two more replaceable hydrogen atoms (two $ OH $ bonds) as clearly depicted from its structure.
$ NaHP{O_3} $ : $ NaHP{O_3} $ is actually formed when $ {H_2}P{O_3} $ reacts with $ NaOH $ in the molar ratio of $ 1:1 $ as shown in the reaction below:
$ NaOH + {H_2}P{O_3} \to NaHP{O_3} + {H_2}O $
Now let’s have a look on the structure of $ NaHP{O_3} $
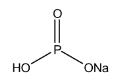
Here we see that $ NaHP{O_3} $ contains one replaceable $ {H^ + } $ ions as one $ OH $ bond is present so it is also considered as an acid salt.
Therefore $ Na{H_2}P{O_4} $ and $ NaHP{O_3} $ are acid salts.
So, the correct option will be Option A i.e. True.
Note:
$ pH $ of acid salts is less than 7 while $ pH $ of the neutral or normal salts usually ranges from $ 5 - 7 $ . Neutral salts are the salts which do not show any acidic or basic nature. If the acid will contain oxygen which are also called as oxoacids then we generally use $ - ous $ and $ - ic $ in the suffix. Few other examples in this category include Hypophosphorous acid or phosphonic acid.
Complete step by step solution:
First let us see what an acid salt is:
Acid salt: An acid is the one which forms an acidic solution when it is dissolved in water. It is formed by partial or incomplete neutralization of acid by a base. An acid salt should contain at least two replaceable hydrogen ions. Let us have a look on $ Na{H_2}P{O_4} $ and $ NaHP{O_3} $ one by one:
$ Na{H_2}P{O_4} $: $ Na{H_2}P{O_4} $ is actually formed when phosphoric acid reacts with sodium hydroxide react in the molar ratio of $ 1:1 $ as shown in the reaction below:
$ NaOH + {H_3}P{O_4} \to Na{H_2}P{O_4} + {H_2}O $
Now let’s have a look on the structure of $ Na{H_2}P{O_4} $

$ Na{H_2}P{O_4} $ is an acid salt because it contains two more replaceable hydrogen atoms (two $ OH $ bonds) as clearly depicted from its structure.
$ NaHP{O_3} $ : $ NaHP{O_3} $ is actually formed when $ {H_2}P{O_3} $ reacts with $ NaOH $ in the molar ratio of $ 1:1 $ as shown in the reaction below:
$ NaOH + {H_2}P{O_3} \to NaHP{O_3} + {H_2}O $
Now let’s have a look on the structure of $ NaHP{O_3} $

Here we see that $ NaHP{O_3} $ contains one replaceable $ {H^ + } $ ions as one $ OH $ bond is present so it is also considered as an acid salt.
Therefore $ Na{H_2}P{O_4} $ and $ NaHP{O_3} $ are acid salts.
So, the correct option will be Option A i.e. True.
Note:
$ pH $ of acid salts is less than 7 while $ pH $ of the neutral or normal salts usually ranges from $ 5 - 7 $ . Neutral salts are the salts which do not show any acidic or basic nature. If the acid will contain oxygen which are also called as oxoacids then we generally use $ - ous $ and $ - ic $ in the suffix. Few other examples in this category include Hypophosphorous acid or phosphonic acid.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




