
When NaCl is dissolved in water, the sodium ion becomes:
A) oxidised
B) reduced
C) hydrolysed
D) hydrated
Answer
582.9k+ views
Hint: We know that, when any salt dissolves in aqueous solvent they break into ions forms one is cationic which has positive charge ion and another is anionic which has negative charge ion. The water molecules get dissociated in hydronium ion and hydroxide ion.
Complete Step by step answer: As we all know, when sodium chloride that is NaCl dissolves in the aqueous solution that is water, usually forms an ion they are the sodium and chloride. The polar water molecules show and are strongly attracted to each other by a famous interaction that is ion-dipole interactions. The solvent molecules that are water are generally surrounded by the ions which are removing them from the salt crystal and easily forming the solution. By means of the dissolving process, the individual ions which are removed from the solid crystal, get completely separate and form hydrated species in the solution.
The salt when dissolved in water forms an ionic compound. Let us consider the diagram of salt NaCl which is dissolved in water given to the ions as shown below.
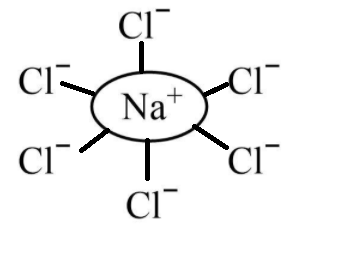
Hence, we can conclude that when NaCl is dissolved in water, the sodium ion becomes hydrated.
Hence, the correct option for this question is D that is hydrated.
Note: The salt in a chemistry can be described by having an ionic form get-together with the cations and the anions. The salts get dissociated with the effect of temperature and changes in the solvent which is needed for dissolving.
Complete Step by step answer: As we all know, when sodium chloride that is NaCl dissolves in the aqueous solution that is water, usually forms an ion they are the sodium and chloride. The polar water molecules show and are strongly attracted to each other by a famous interaction that is ion-dipole interactions. The solvent molecules that are water are generally surrounded by the ions which are removing them from the salt crystal and easily forming the solution. By means of the dissolving process, the individual ions which are removed from the solid crystal, get completely separate and form hydrated species in the solution.
The salt when dissolved in water forms an ionic compound. Let us consider the diagram of salt NaCl which is dissolved in water given to the ions as shown below.

Hence, we can conclude that when NaCl is dissolved in water, the sodium ion becomes hydrated.
Hence, the correct option for this question is D that is hydrated.
Note: The salt in a chemistry can be described by having an ionic form get-together with the cations and the anions. The salts get dissociated with the effect of temperature and changes in the solvent which is needed for dissolving.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction




