
$N{{a}_{2}}{{S}_{2}}{{O}_{3}}$ is used in photography for fixing the negative. It removes AgBr by:
(A) Formation of the complex ( $[N{{a}_{3}}{{(Ag{{S}_{2}}{{O}_{3}})}_{2}}]$ )
(B) Oxidation of $B{{r}^{-}}$ to $B{{r}_{2}}$
(C) Reduction of $A{{g}^{+}}$ to Ag
(D) Formation of double salt
Answer
578.4k+ views
Hint: A chemical compound that has a formula $N{{a}_{2}}{{S}_{2}}{{O}_{3}}$ is named sodium thiosulfate. The commercial name of sodium thiosulfate is called Hypo. It is a crystalline solid that tends to readily lose water and readily soluble in water is referred to as sodium thiosulfate.
Complete step by step solution:
Sodium thiosulfate structure:
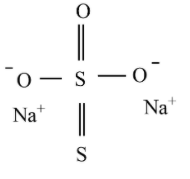
The shape of the thiosulfate ion is tetrahedral in the solid-state of sodium thiosulfate. Compared to thiosulfate ion, the distance between two sigma bonded sulfur atoms differs from the distance between the two sulfur atoms which implies that the sulfur is not bonded to any oxygen that holds a negative charge.
Physical and chemical properties of $N{{a}_{2}}{{S}_{2}}{{O}_{3}}$ :
- Sodium thiosulfate has a white crystalline solid and is odorless.
- The density of hypo is 1.667 grams per cubic meter.
- The solubility of hypo in water is 70.1 g/100 ml at ${{20}^{o}}C$ and 231g/100ml at ${{100}^{o}}C$
- The crystal structure of sodium thiosulfate crystals is monoclinic.
$N{{a}_{2}}{{S}_{2}}{{O}_{3}}$ is used in photography for fixing the negative. It removes AgBr by Formation of the complex ( $[N{{a}_{3}}{{(Ag{{S}_{2}}{{O}_{3}})}_{2}}]$ ) which is an undecomposed complex. The reaction of the process as follows:
$AgBr+2N{{a}_{2}}{{S}_{2}}{{O}_{3}}\to N{{a}_{3}}[A{{g}_{2}}{{({{S}_{2}}{{O}_{3}})}_{2}}]+NaBr$
Hence, the correct answer is option A.
Note: Sodium thiosulfate causes mild skin irritation, mechanical eye irritation, and causes upper respiratory tract irritation. It is used in the medical treatment of cyanide poisoning cases and used in the treatment for the side effects of chemotherapy and hemodialysis.
Complete step by step solution:
Sodium thiosulfate structure:

The shape of the thiosulfate ion is tetrahedral in the solid-state of sodium thiosulfate. Compared to thiosulfate ion, the distance between two sigma bonded sulfur atoms differs from the distance between the two sulfur atoms which implies that the sulfur is not bonded to any oxygen that holds a negative charge.
Physical and chemical properties of $N{{a}_{2}}{{S}_{2}}{{O}_{3}}$ :
- Sodium thiosulfate has a white crystalline solid and is odorless.
- The density of hypo is 1.667 grams per cubic meter.
- The solubility of hypo in water is 70.1 g/100 ml at ${{20}^{o}}C$ and 231g/100ml at ${{100}^{o}}C$
- The crystal structure of sodium thiosulfate crystals is monoclinic.
$N{{a}_{2}}{{S}_{2}}{{O}_{3}}$ is used in photography for fixing the negative. It removes AgBr by Formation of the complex ( $[N{{a}_{3}}{{(Ag{{S}_{2}}{{O}_{3}})}_{2}}]$ ) which is an undecomposed complex. The reaction of the process as follows:
$AgBr+2N{{a}_{2}}{{S}_{2}}{{O}_{3}}\to N{{a}_{3}}[A{{g}_{2}}{{({{S}_{2}}{{O}_{3}})}_{2}}]+NaBr$
Hence, the correct answer is option A.
Note: Sodium thiosulfate causes mild skin irritation, mechanical eye irritation, and causes upper respiratory tract irritation. It is used in the medical treatment of cyanide poisoning cases and used in the treatment for the side effects of chemotherapy and hemodialysis.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




