
n: When $but - 2 - yne$ is treated with $dil.\,{H_2}S{O_4},\,HgS{O_4}$ the product formed is:
A.Butan $ - 1 - ol$
B.Butan $ - 2 - ol$
C.Acetone
D.Butanone
Answer
547.2k+ views
Hint: Don’t get confused after seeing only sulphuric acid that it is a dehydrogenation reaction as it is not. See that the reagent is a mixture of both sulphuric acid and mercuric sulphate, we get to know all the steps required for the reaction. Start by converting alkyne into alcohol then try to solve it further.
Complete step-by-step answer:In the first step see that it is oxymercuration- demercuration reaction in which there is an introduction of alcoholic group at carbon position around double or triple bond according to Markonikov rule. Here as you are seeing that the substrate is symmetrical from both sides for introduction of alcoholic groups. So in the first step as you see in the reaction, triple bonds get changed into double bonds and there is an introduction of alcoholic groups. We can write the equation as
\[C{H_3} - C \equiv C - C{H_3}\,\, + \,{H_2}O\xrightarrow{{H{g^{ + 2}}}}\,C{H_3} - C(H) = C(OH) - C{H_3}\] and
\[C{H_3} - C(H) = C(OH) - C{H_3}\xrightarrow{{Oxidation\,}}\,C{H_3} - C{H_2} - (C = O) - C{H_3}\]
We can also write the same equations as given in the following diagram where the formation of alcohol is done after oxidation ketone is formed.
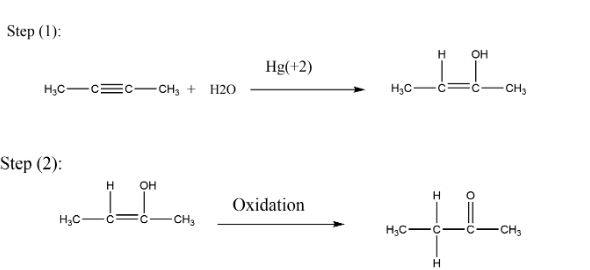
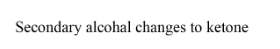
As it is given sulphuric acid so here in the next step oxidation takes place, for the oxidation process there is a carbonyl group (ketone) formed having two alkyl groups around it so we can call it as ketone. For ketone, (-one) is used at last so the resulting compound is butanone and nothing else. Thus secondary alcohol changes to ketone, if we are given with primary alcohol then on oxidation it gets converted to aldehyde.
Note:You can understand the whole oxidation process by sulphuric acid using a mechanism, in which firstly hydrogen ions get attracted to the lone pair of electrons. After it tautomerism takes place in which whenever we are having a double bond and $(OH)$ group by side they rearrange themselves and form $( - C = O)$ that is a carbonyl group.
Complete step-by-step answer:In the first step see that it is oxymercuration- demercuration reaction in which there is an introduction of alcoholic group at carbon position around double or triple bond according to Markonikov rule. Here as you are seeing that the substrate is symmetrical from both sides for introduction of alcoholic groups. So in the first step as you see in the reaction, triple bonds get changed into double bonds and there is an introduction of alcoholic groups. We can write the equation as
\[C{H_3} - C \equiv C - C{H_3}\,\, + \,{H_2}O\xrightarrow{{H{g^{ + 2}}}}\,C{H_3} - C(H) = C(OH) - C{H_3}\] and
\[C{H_3} - C(H) = C(OH) - C{H_3}\xrightarrow{{Oxidation\,}}\,C{H_3} - C{H_2} - (C = O) - C{H_3}\]
We can also write the same equations as given in the following diagram where the formation of alcohol is done after oxidation ketone is formed.

As it is given sulphuric acid so here in the next step oxidation takes place, for the oxidation process there is a carbonyl group (ketone) formed having two alkyl groups around it so we can call it as ketone. For ketone, (-one) is used at last so the resulting compound is butanone and nothing else. Thus secondary alcohol changes to ketone, if we are given with primary alcohol then on oxidation it gets converted to aldehyde.
Note:You can understand the whole oxidation process by sulphuric acid using a mechanism, in which firstly hydrogen ions get attracted to the lone pair of electrons. After it tautomerism takes place in which whenever we are having a double bond and $(OH)$ group by side they rearrange themselves and form $( - C = O)$ that is a carbonyl group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




