
n – propyl alcohol and isopropyl alcohol are examples of:
A.Position isomerism
B.Chain isomerism
C.Tautomerism
D.Geometrical isomerism
Answer
578.1k+ views
Hint: Isomerism can be understood as the phenomenon when two or more compounds have the same molecular formula, but they have different molecular structures. To explain this in simpler terms, we can say that two or more compounds have the same constituent atoms in equal numbers, but the arrangement of these atoms within the respective molecules are different.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The compounds given to us are n – propyl alcohol and isopropyl alcohol and their molecular structures can be given as:
1.n – propyl alcohol
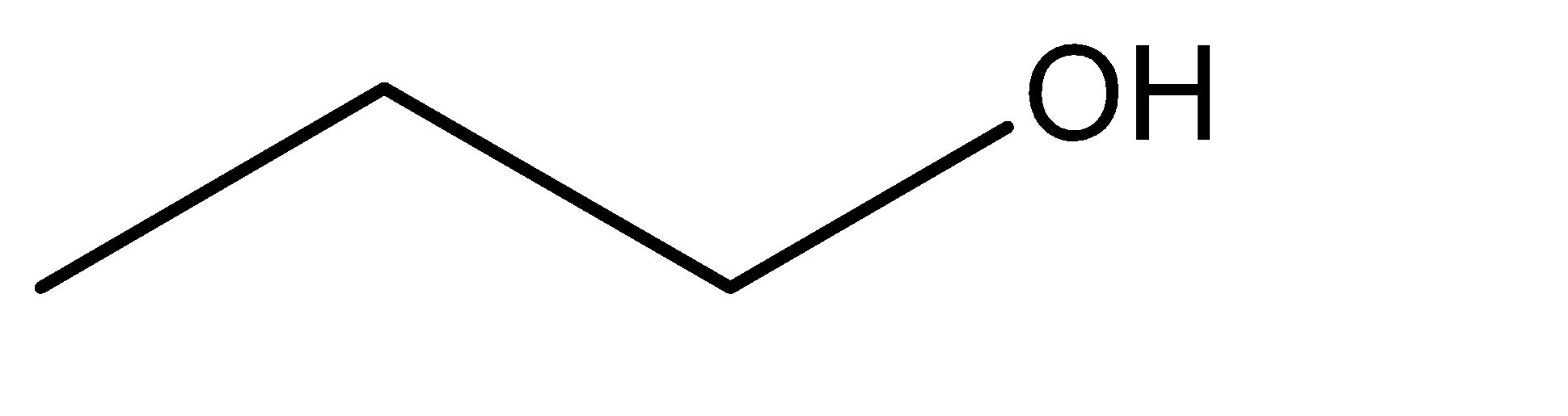
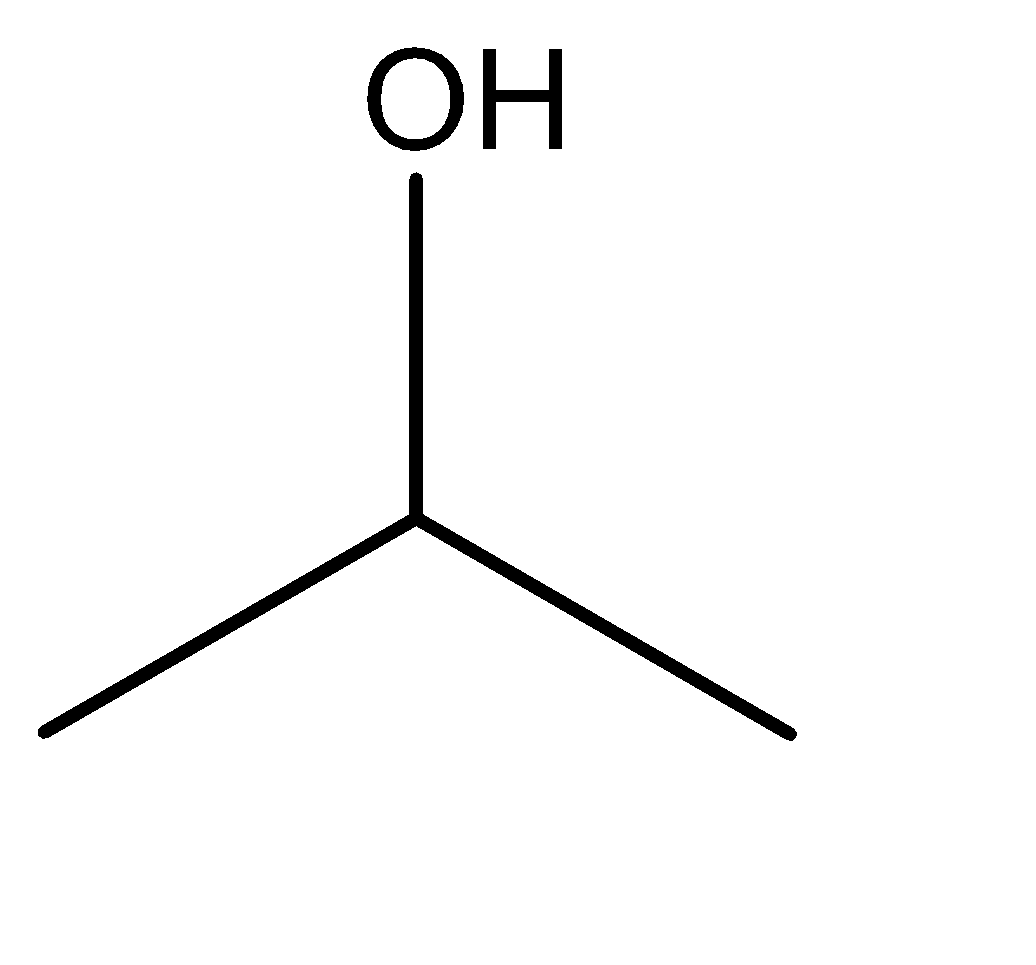
2.isopropyl alcohol
Now, Tautomerism can be explained as a type of isomerism that is exhibited by different compounds which involves only the change in the positions of protons and electrons, rather than the entire molecular structure. This means that positions of the constituent atoms are unaltered but the orientations of the electrons and protons are shifting.
Chain isomerism can be explained as the variation in the arrangements of the carbon chains within the given molecules. To explain it in simpler terms, we can explain chain isomers as two or more molecules which have the same molecular formula but different parent chains in their molecular structure.
Position isomerism occurs when two or more molecules have the same functional groups present on different positions in the same parent carbon chain
Geometrical isomerism is a type of isomerism where the compounds under consideration have the same structural formulae but the arrangements of the groups of attached substituents may differ. These variations may occur in groups of single atoms, in rings or even in the double bonds present in the structure.
Hence, from the discussion above and the molecular structures of the given compounds, we can say the isomerism exhibited in this case is position isomerism
Hence, Option A is the correct option
Note: The major condition for any compound to exhibit tautomerism is that it should have at least one alpha hydrogen atom. In tautomerism, molecules with the same molecular formula interconvert rapidly. Hence, these molecules also exhibit dynamic equilibrium with each other. Also, tautomerism can be exhibited by any functional group as long as the parent chain has at least one alpha hydrogen.
Complete Step-by-Step Answer:
Before we move forward with the solution of the given question, let us first understand some important basic concepts.
The compounds given to us are n – propyl alcohol and isopropyl alcohol and their molecular structures can be given as:
1.n – propyl alcohol

2.isopropyl alcohol

Now, Tautomerism can be explained as a type of isomerism that is exhibited by different compounds which involves only the change in the positions of protons and electrons, rather than the entire molecular structure. This means that positions of the constituent atoms are unaltered but the orientations of the electrons and protons are shifting.
Chain isomerism can be explained as the variation in the arrangements of the carbon chains within the given molecules. To explain it in simpler terms, we can explain chain isomers as two or more molecules which have the same molecular formula but different parent chains in their molecular structure.
Position isomerism occurs when two or more molecules have the same functional groups present on different positions in the same parent carbon chain
Geometrical isomerism is a type of isomerism where the compounds under consideration have the same structural formulae but the arrangements of the groups of attached substituents may differ. These variations may occur in groups of single atoms, in rings or even in the double bonds present in the structure.
Hence, from the discussion above and the molecular structures of the given compounds, we can say the isomerism exhibited in this case is position isomerism
Hence, Option A is the correct option
Note: The major condition for any compound to exhibit tautomerism is that it should have at least one alpha hydrogen atom. In tautomerism, molecules with the same molecular formula interconvert rapidly. Hence, these molecules also exhibit dynamic equilibrium with each other. Also, tautomerism can be exhibited by any functional group as long as the parent chain has at least one alpha hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




