
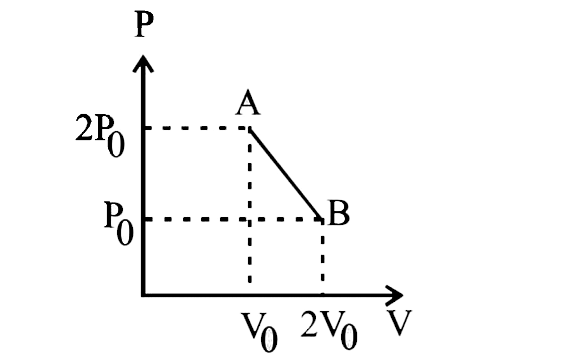
‘n’ moles of ideal gas undergo a process \[A\to B\] as shown in the figure. The maximum temperature of the gas during the process will be:
A. \[\dfrac{9{{P}_{0}}{{V}_{0}}}{4nR}\]
B. \[\dfrac{3{{P}_{0}}{{V}_{0}}}{2nR}\]
C.\[\dfrac{9{{P}_{0}}{{V}_{0}}}{2nR}\]
D. \[\dfrac{9{{P}_{0}}{{V}_{0}}}{nR}\]

Answer
578.1k+ views
Hint: Two points of the P-V graph have been given. Two-point form of a line can be used to find the formula of the P-V line of the process. We also know from the ideal gas equation that the temperature of the gas is related to pressure and volume as given below. We also know that the for the temperature to be maximum \[\dfrac{dT}{dV}=0\]
Formula used:
\[T=\dfrac{PV}{nR}\]
Complete answer:
We have been given the pressure vs volume graph. The P-V graph of the given process is a straight line as can be seen from the diagram. Two point of the graph, \[\left( {{V}_{0}},2{{P}_{0}} \right)\]\[(2{{V}_{0}},{{P}_{0}})\] are also given.
So, the equation of the P-V graph in two-point form would be
\[\begin{align}
& \left( P-{{P}_{0}} \right)=\dfrac{\left( 2{{P}_{0}}-{{P}_{0}} \right)}{\left( {{V}_{0}}-2{{V}_{0}} \right)}\left( V-2{{V}_{0}} \right)=\dfrac{{{P}_{0}}}{{{V}_{0}}}\left( 2{{V}_{0}}-V \right) \\
& \Rightarrow P=3{{P}_{0}}-\dfrac{{{P}_{0}}V}{{{V}_{0}}} \\
\end{align}\]
So the equation of the graph is \[P=3{{P}_{0}}-\dfrac{{{P}_{0}}V}{{{V}_{0}}}\]…….. (1)
From the ideal gas equation, we know that
\[T=\dfrac{PV}{nR}\]
So the temperature of the given process can be found by substituting value of P in (1)
\[\begin{align}
& T=\dfrac{PV}{nR} \\
& \Rightarrow T=\left( 3{{P}_{0}}-\dfrac{{{P}_{0}}V}{{{V}_{0}}} \right)\dfrac{V}{nR} \\
& \Rightarrow T=\dfrac{3{{P}_{0}}{{V}_{0}}V-{{P}_{0}}{{V}^{2}}}{nR{{V}_{0}}} \\
\end{align}\]
So, Temperature at Volume V is given by \[T=\dfrac{3{{P}_{0}}{{V}_{0}}V-{{P}_{0}}{{V}^{2}}}{nR{{V}_{0}}}\]….(2)
We also know that for the temperature to be maximum \[\dfrac{dT}{dV}=0\]
\[\dfrac{dT}{dV}=\dfrac{d\left( \dfrac{3{{P}_{0}}{{V}_{0}}V-{{P}_{0}}{{V}^{2}}}{nR{{V}_{0}}} \right)}{dV}\] should be 0 for the temperature to be maximum
\[\begin{align}
& \dfrac{dT}{dV}=\dfrac{d\left( \dfrac{3{{P}_{0}}{{V}_{0}}V-{{P}_{0}}{{V}^{2}}}{nR{{V}_{0}}} \right)}{dV}=0 \\
& \Rightarrow 3{{P}_{0}}{{V}_{0}}-2{{P}_{0}}V=0 \\
& \Rightarrow V=\dfrac{3{{V}_{0}}}{2} \\
\end{align}\]
So the temperature will be maximum at \[V=\dfrac{3{{V}_{0}}}{2}\]
Putting this value of V in (2) we get
\[\begin{align}
& {{T}_{max}}=\dfrac{3{{P}_{0}}{{V}_{0}}\left( \dfrac{3{{V}_{0}}}{2} \right)-{{P}_{0}}\left( \dfrac{9}{4}{{V}_{0}}^{2} \right)}{nR{{V}_{0}}} \\
& \Rightarrow {{T}_{max}}=\dfrac{9{{P}_{0}}{{V}_{0}}}{4nR} \\
\end{align}\]
So, the maximum temperature of the gas during the process will be \[\dfrac{9{{P}_{0}}{{V}_{0}}}{4nR}\]
So, the correct answer is “Option A”.
Note:
The ideal gas equation is arguably the most important equation in Gaseous State and thermodynamics. It is the relation between pressure, volume, temperature, and the number of moles of an ideal gas undergoing a process. It is given as \[PV=nRT\]. For real gases the equation has a few correctional modifications and the modified equation for real gases is given as \[\left( P+\dfrac{a{{n}^{2}}}{{{V}^{2}}} \right)(V-nb)=nRT\]. This equation is called Van der Waals Equation.
Formula used:
\[T=\dfrac{PV}{nR}\]
Complete answer:
We have been given the pressure vs volume graph. The P-V graph of the given process is a straight line as can be seen from the diagram. Two point of the graph, \[\left( {{V}_{0}},2{{P}_{0}} \right)\]\[(2{{V}_{0}},{{P}_{0}})\] are also given.
So, the equation of the P-V graph in two-point form would be
\[\begin{align}
& \left( P-{{P}_{0}} \right)=\dfrac{\left( 2{{P}_{0}}-{{P}_{0}} \right)}{\left( {{V}_{0}}-2{{V}_{0}} \right)}\left( V-2{{V}_{0}} \right)=\dfrac{{{P}_{0}}}{{{V}_{0}}}\left( 2{{V}_{0}}-V \right) \\
& \Rightarrow P=3{{P}_{0}}-\dfrac{{{P}_{0}}V}{{{V}_{0}}} \\
\end{align}\]
So the equation of the graph is \[P=3{{P}_{0}}-\dfrac{{{P}_{0}}V}{{{V}_{0}}}\]…….. (1)
From the ideal gas equation, we know that
\[T=\dfrac{PV}{nR}\]
So the temperature of the given process can be found by substituting value of P in (1)
\[\begin{align}
& T=\dfrac{PV}{nR} \\
& \Rightarrow T=\left( 3{{P}_{0}}-\dfrac{{{P}_{0}}V}{{{V}_{0}}} \right)\dfrac{V}{nR} \\
& \Rightarrow T=\dfrac{3{{P}_{0}}{{V}_{0}}V-{{P}_{0}}{{V}^{2}}}{nR{{V}_{0}}} \\
\end{align}\]
So, Temperature at Volume V is given by \[T=\dfrac{3{{P}_{0}}{{V}_{0}}V-{{P}_{0}}{{V}^{2}}}{nR{{V}_{0}}}\]….(2)
We also know that for the temperature to be maximum \[\dfrac{dT}{dV}=0\]
\[\dfrac{dT}{dV}=\dfrac{d\left( \dfrac{3{{P}_{0}}{{V}_{0}}V-{{P}_{0}}{{V}^{2}}}{nR{{V}_{0}}} \right)}{dV}\] should be 0 for the temperature to be maximum
\[\begin{align}
& \dfrac{dT}{dV}=\dfrac{d\left( \dfrac{3{{P}_{0}}{{V}_{0}}V-{{P}_{0}}{{V}^{2}}}{nR{{V}_{0}}} \right)}{dV}=0 \\
& \Rightarrow 3{{P}_{0}}{{V}_{0}}-2{{P}_{0}}V=0 \\
& \Rightarrow V=\dfrac{3{{V}_{0}}}{2} \\
\end{align}\]
So the temperature will be maximum at \[V=\dfrac{3{{V}_{0}}}{2}\]
Putting this value of V in (2) we get
\[\begin{align}
& {{T}_{max}}=\dfrac{3{{P}_{0}}{{V}_{0}}\left( \dfrac{3{{V}_{0}}}{2} \right)-{{P}_{0}}\left( \dfrac{9}{4}{{V}_{0}}^{2} \right)}{nR{{V}_{0}}} \\
& \Rightarrow {{T}_{max}}=\dfrac{9{{P}_{0}}{{V}_{0}}}{4nR} \\
\end{align}\]
So, the maximum temperature of the gas during the process will be \[\dfrac{9{{P}_{0}}{{V}_{0}}}{4nR}\]
So, the correct answer is “Option A”.
Note:
The ideal gas equation is arguably the most important equation in Gaseous State and thermodynamics. It is the relation between pressure, volume, temperature, and the number of moles of an ideal gas undergoing a process. It is given as \[PV=nRT\]. For real gases the equation has a few correctional modifications and the modified equation for real gases is given as \[\left( P+\dfrac{a{{n}^{2}}}{{{V}^{2}}} \right)(V-nb)=nRT\]. This equation is called Van der Waals Equation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




