
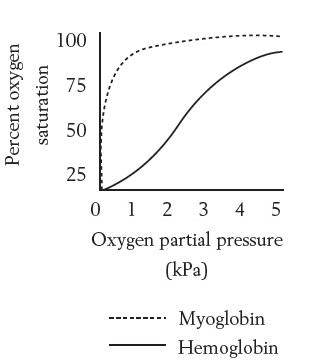
Myoglobin is expressed in muscle cells. The difference between the binding of oxygen to hemoglobin and myoglobin can be best explained by the fact that
A. Hemoglobin must retain oxygen in the veins.
B. Hemoglobin must remove oxygen from oxygen-poor air.
C. Myoglobin must be able to transport oxygen to the brain.
D. Myoglobin must be able to remove oxygen from hemoglobin
E. Hemoglobin must bind oxygen even in the presence of myoglobin

Answer
498.6k+ views
Hint: Haemoglobin is defined as an oxygen-carrying pigment and predominant protein present in red blood cells. It is an iron containing protein in the blood, which transports oxygen to tissues.
Myoglobin is defined as a red protein containing haem, which carries and stores oxygen in muscle cells. It is an oxygen-binding protein, located primarily in muscle cells.
Complete answer:
Hemoglobin is a tetramer, while myoglobin is a monomeric protein. So, myoglobin can bind to one molecule of oxygen and hemoglobin can bind to four molecules of oxygen. Hemoglobin is present in red blood cells, whereas myoglobin is present in muscle tissue. Both hemoglobin and myoglobin vary in many amino acids in their primary structures.
Option A Hemoglobin must retain oxygen in the veins: In the veins, oxygen is not present as impure blood flows through the veins.
Option A is not correct
Option B Hemoglobin must remove air from oxygen-poor air: When carbon contents are present in the air, hemoglobin drops off the oxygen from it.
Hence, Option B is not correct
Option C Myoglobin must be able to transport oxygen to the brain: Myoglobin serves a local temporary reservoir of oxygen, which can carry oxygen to blood.
So, Option C is not correct
Option D Myoglobin must be able to remove oxygen from hemoglobin: Myoglobin consists of a heme prosthetic group, which can reversibly bind to oxygen. It has more affinity to oxygen from hemoglobin. So it is able to remove oxygen from hemoglobin.
Option D is correct
Option E Hemoglobin must bind oxygen even in the presence of myoglobin: Since myoglobin displaces hemoglobin from oxygen, hemoglobin is not able to bind oxygen in the presence of myoglobin.
Option E is not correct
Option D is the correct answer.
Note:
Haemoglobin is a transport protein. But, myoglobin is an intracellular storage site for oxygen. It binds tighter than hemoglobin due to more affinity The difference in affinity is due to the fact that myoglobin does not have a quaternary structure, as in the case of hemoglobin. The amino acids changes are conservative between two hemoglobin and myoglobin.
Myoglobin is defined as a red protein containing haem, which carries and stores oxygen in muscle cells. It is an oxygen-binding protein, located primarily in muscle cells.
Complete answer:
Hemoglobin is a tetramer, while myoglobin is a monomeric protein. So, myoglobin can bind to one molecule of oxygen and hemoglobin can bind to four molecules of oxygen. Hemoglobin is present in red blood cells, whereas myoglobin is present in muscle tissue. Both hemoglobin and myoglobin vary in many amino acids in their primary structures.
Option A Hemoglobin must retain oxygen in the veins: In the veins, oxygen is not present as impure blood flows through the veins.
Option A is not correct
Option B Hemoglobin must remove air from oxygen-poor air: When carbon contents are present in the air, hemoglobin drops off the oxygen from it.
Hence, Option B is not correct
Option C Myoglobin must be able to transport oxygen to the brain: Myoglobin serves a local temporary reservoir of oxygen, which can carry oxygen to blood.
So, Option C is not correct
Option D Myoglobin must be able to remove oxygen from hemoglobin: Myoglobin consists of a heme prosthetic group, which can reversibly bind to oxygen. It has more affinity to oxygen from hemoglobin. So it is able to remove oxygen from hemoglobin.
Option D is correct
Option E Hemoglobin must bind oxygen even in the presence of myoglobin: Since myoglobin displaces hemoglobin from oxygen, hemoglobin is not able to bind oxygen in the presence of myoglobin.
Option E is not correct
Option D is the correct answer.
Note:
Haemoglobin is a transport protein. But, myoglobin is an intracellular storage site for oxygen. It binds tighter than hemoglobin due to more affinity The difference in affinity is due to the fact that myoglobin does not have a quaternary structure, as in the case of hemoglobin. The amino acids changes are conservative between two hemoglobin and myoglobin.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




