
Multiple covalent bonds exist in a molecule of?
Option-
A.${F_2}$
B.${H_2}$
C.${N_2}$
D.${C_2}{H_6}$
Answer
497.4k+ views
Hint: Covalent bond is formed by the process of mutual sharing of valence shell electrons of combining atoms which may be the same or from different elements. Covalent bond is of three types: single bond, double bond or triple bond.
Complete answer:
Covalent bonds which are double bond or triple bond are collectively known as multiple covalent bonds because they contain more than one covalent bond. Double bond is formed between two atoms by the sharing of two electron pairs, and it is represented as $\left( = \right)$. Triple bond formed between two atoms by sharing three electron pairs, and it is represented as $\left( \equiv \right)$.
In the molecule of ${F_2}$ two fluorine atoms are bonded with each other. As we know fluorine has seven electrons in its outermost shell and requires only one more electron to attain noble gas configuration neon. So, each fluorine atom shares one electron with each other to form a single bond.
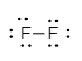
In the molecule of ${H_2}$ two hydrogen atoms are bonded with each other. As we know hydrogen has one electron in its outermost shell and requires only one more electron to attain noble gas configuration of helium. So, each hydrogen atom shares one electron with each other to form a single bond.
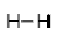
In the molecule of ${N_2}$ two nitrogen atoms are bonded with each other. As we know nitrogen has five electrons in its outermost shell and requires three more electrons to attain noble gas configuration of neon. So, each nitrogen atom shares three electrons with each other to form a triple bond.

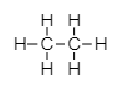
In the molecule of ${C_2}{H_6}$ two carbon atoms are bonded with each other. As we know carbon has four electrons in its outermost shell and requires four more electrons to attain noble gas configuration of neon. So, each carbon atom shares one electron with another carbon atom to form a single bond. And the remaining three electrons are shared with hydrogen atoms. ${C_2}{H_6}$ is a saturated organic compound with single covalent bonds only.
$ \Rightarrow$ molecule of ${N_2}$ contains multiple covalent bonds.
Therefore, option $\left( C \right)$ is the correct option.
Note:
Double and triple bond easily undergoes the reaction of addition while single bond undergoes substitution mainly. Bond length of the atom decreases as we move from single bond to double bond and finally shortest to triple bond. However, the reactivity of the triple bond is maximum.
Complete answer:
Covalent bonds which are double bond or triple bond are collectively known as multiple covalent bonds because they contain more than one covalent bond. Double bond is formed between two atoms by the sharing of two electron pairs, and it is represented as $\left( = \right)$. Triple bond formed between two atoms by sharing three electron pairs, and it is represented as $\left( \equiv \right)$.
In the molecule of ${F_2}$ two fluorine atoms are bonded with each other. As we know fluorine has seven electrons in its outermost shell and requires only one more electron to attain noble gas configuration neon. So, each fluorine atom shares one electron with each other to form a single bond.
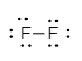
In the molecule of ${H_2}$ two hydrogen atoms are bonded with each other. As we know hydrogen has one electron in its outermost shell and requires only one more electron to attain noble gas configuration of helium. So, each hydrogen atom shares one electron with each other to form a single bond.
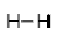
In the molecule of ${N_2}$ two nitrogen atoms are bonded with each other. As we know nitrogen has five electrons in its outermost shell and requires three more electrons to attain noble gas configuration of neon. So, each nitrogen atom shares three electrons with each other to form a triple bond.

In the molecule of ${C_2}{H_6}$ two carbon atoms are bonded with each other. As we know carbon has four electrons in its outermost shell and requires four more electrons to attain noble gas configuration of neon. So, each carbon atom shares one electron with another carbon atom to form a single bond. And the remaining three electrons are shared with hydrogen atoms. ${C_2}{H_6}$ is a saturated organic compound with single covalent bonds only.

$ \Rightarrow$ molecule of ${N_2}$ contains multiple covalent bonds.
Therefore, option $\left( C \right)$ is the correct option.
Note:
Double and triple bond easily undergoes the reaction of addition while single bond undergoes substitution mainly. Bond length of the atom decreases as we move from single bond to double bond and finally shortest to triple bond. However, the reactivity of the triple bond is maximum.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




