
Mononitration of phenyl benzoate gives which has the major product:
(A)
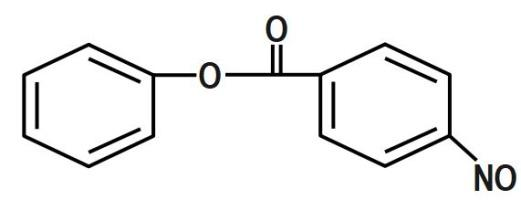
(B)
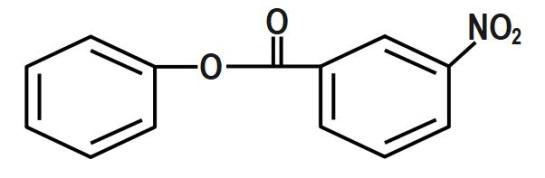
(C)
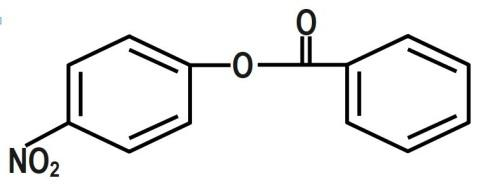
(D)
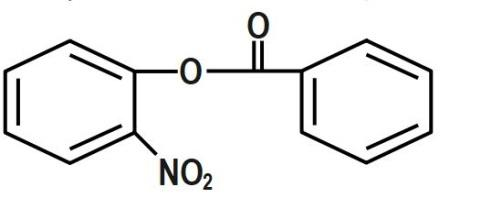




Answer
514.2k+ views
Hint : We know that the presence of electron withdrawing groups such as $ N{{O}_{2}},\text{ }CN $ etc. at ortho and para positions but not at m-positions with respect to the halogen greatly activates the halogen towards nucleophilic displacement. We also know that the number of such groups at ortho and para positions with respect to the halogens vary directly with the reactivity of the haloarene.
Complete Step By Step Answer:
We know that the ring which is attached to the $ O- $ atom, in which the nitro group enters and as the result of its group gets activated. It is known that it will not get into the ring to which the carbonyl group as it will deactivate the group. We can now say that the group is ortho and para directing in nature. The chemical equation that represents the formation of $ 4- $ nitrophenyl benzoate is shown as follows:
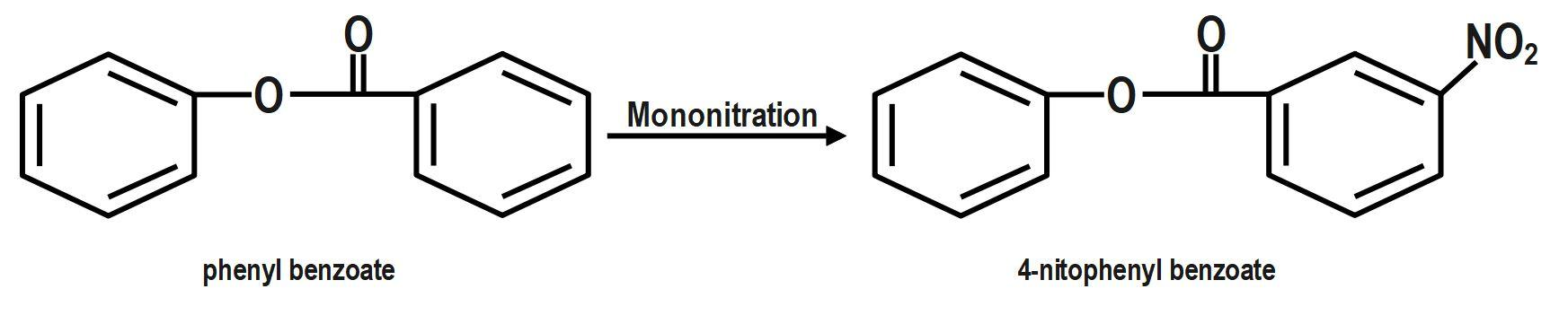
Nitration of phenyl benzoate yields $ 4- $ nitrophenyl benzoate. Nitro group will enter the ring that is attached to ortho atom as $ -O-C\left( =O \right)-{{C}_{6}}{{H}_{5}} $ is activating group. It will not enter the ring attached to carbonyl group as $ -C\left( =O \right)-O-{{C}_{6}}{{H}_{5}} $ is deactivating group. But here $ -O-C\left( =O \right)-{{C}_{6}}{{H}_{5}} $ is ortho and para director. It goes to the para position because the Benzene ring will be unstable if the $ N{{O}_{2}} $ group is attached anywhere else. It is an electron donating group and also an unstable compound at Meta or Ortho positions.
Therefore, the correct answer is option B.
Note :
Note that the phenyl benzoate is a compound having very little life and hence it is very unstable. Its nitration is not feasible to carry out. But still if nitration is carried out then it will give a Meta product. In such types of problems, the properties of compounds are very important.
Complete Step By Step Answer:
We know that the ring which is attached to the $ O- $ atom, in which the nitro group enters and as the result of its group gets activated. It is known that it will not get into the ring to which the carbonyl group as it will deactivate the group. We can now say that the group is ortho and para directing in nature. The chemical equation that represents the formation of $ 4- $ nitrophenyl benzoate is shown as follows:

Nitration of phenyl benzoate yields $ 4- $ nitrophenyl benzoate. Nitro group will enter the ring that is attached to ortho atom as $ -O-C\left( =O \right)-{{C}_{6}}{{H}_{5}} $ is activating group. It will not enter the ring attached to carbonyl group as $ -C\left( =O \right)-O-{{C}_{6}}{{H}_{5}} $ is deactivating group. But here $ -O-C\left( =O \right)-{{C}_{6}}{{H}_{5}} $ is ortho and para director. It goes to the para position because the Benzene ring will be unstable if the $ N{{O}_{2}} $ group is attached anywhere else. It is an electron donating group and also an unstable compound at Meta or Ortho positions.
Therefore, the correct answer is option B.
Note :
Note that the phenyl benzoate is a compound having very little life and hence it is very unstable. Its nitration is not feasible to carry out. But still if nitration is carried out then it will give a Meta product. In such types of problems, the properties of compounds are very important.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




