
Mono sodium acetylide reacts with an alkyl halide to form:
(A) An alkane
(B) An alkene
(C) an unsymmetrical higher alkyne
(D) a symmetrical higher alkyne
Answer
583.8k+ views
Hint: As we know that acetylene contains two hydrogen and two carbon in which carbon is attached with another carbon with triple bond if sodium is attached with acetylene then it will act as a nucleophile.
Complete step by step answer:
The mono sodium acetylide is prepared by sodium hydroxide (a strong base). When acetylene reacts with sodium hydroxide, this abstracts the hydrogen from acetylene because the acetylene proton is acidic due to \[sp\] hybridization of carbon thus, water eliminates and we get mono sodium acetylide which is represented as \[CH \equiv {C^ - }N{a^ + }\].
Now \[CH \equiv {C^ - }N{a^ + }\] contains a dipolar group (ylide) and acts as a nucleophile so it reacts with alkyl halide by \[S{N^2}\] (nucleophilic substitution reaction) process. The alkyl halide contains a halogen element which is a good leaving group that can easily leave when\[CH \equiv {C^ - }N{a^ + }\] attacks on it.
As we can see in the mechanism-
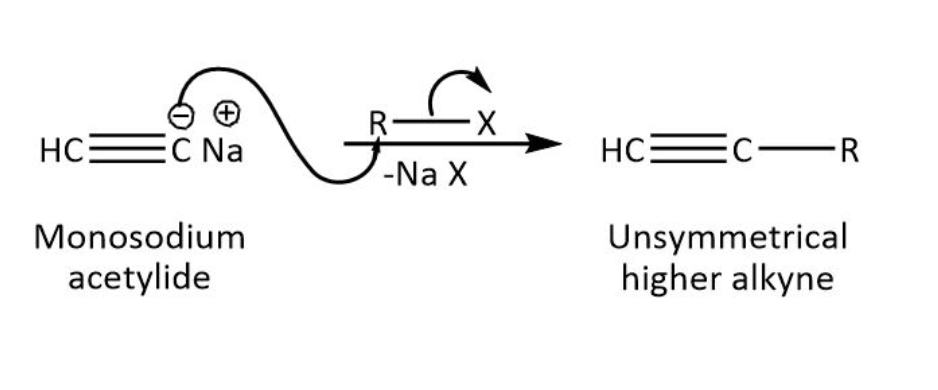
Therefore, the alkyl group is attached with the nucleophilic carbon by eliminating sodium halide.
Now the product is formed which contains an alkyne group and alkyl group so the correct option is option (C).
Note:
The symmetrical higher alkyne can also be prepared with the above mechanism but we have to take two equivalents of sodium hydroxide.
Complete step by step answer:
The mono sodium acetylide is prepared by sodium hydroxide (a strong base). When acetylene reacts with sodium hydroxide, this abstracts the hydrogen from acetylene because the acetylene proton is acidic due to \[sp\] hybridization of carbon thus, water eliminates and we get mono sodium acetylide which is represented as \[CH \equiv {C^ - }N{a^ + }\].
Now \[CH \equiv {C^ - }N{a^ + }\] contains a dipolar group (ylide) and acts as a nucleophile so it reacts with alkyl halide by \[S{N^2}\] (nucleophilic substitution reaction) process. The alkyl halide contains a halogen element which is a good leaving group that can easily leave when\[CH \equiv {C^ - }N{a^ + }\] attacks on it.
As we can see in the mechanism-

Therefore, the alkyl group is attached with the nucleophilic carbon by eliminating sodium halide.
Now the product is formed which contains an alkyne group and alkyl group so the correct option is option (C).
Note:
The symmetrical higher alkyne can also be prepared with the above mechanism but we have to take two equivalents of sodium hydroxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




