
Molecular weight of phorone is equal to:
(A) 2 molecular weight of acetone – molecular weight of water
(B) 3 molecular weight of acetone – 2 molecular weight of water
(C) 3 molecular weight of acetone – molecular weight of water
(D) 2 molecular weight of acetone – 2 molecular weight of water
Answer
547.5k+ views
Hint: To answer this question, you must recall the structure and formation of phorone. Also you must know the molecular mass of acetone and water to be able to calculate the molecular weight of phorone with respect to them.
Complete step by step solution:
Phorone is the common name for the compound diIsopropylidene acetone. It is a crystalline yellow coloured substance and it has an odour like that of germanium. Phorone is a ketone with double bonds attached on both of its alpha carbon atoms.
According to IUPAC nomenclature, the name of the compound phorone is 2, 6- Dimethyl 2, 5- heptadien- 4- one. The IUPAC nomenclature of organic compounds has set rules in such a way that each organic compound has a unique name.
Knowing the IUPAC name of phorone as 2, 6- Dimethyl 2, 5- heptadien- 4- one, we can draw its structure as
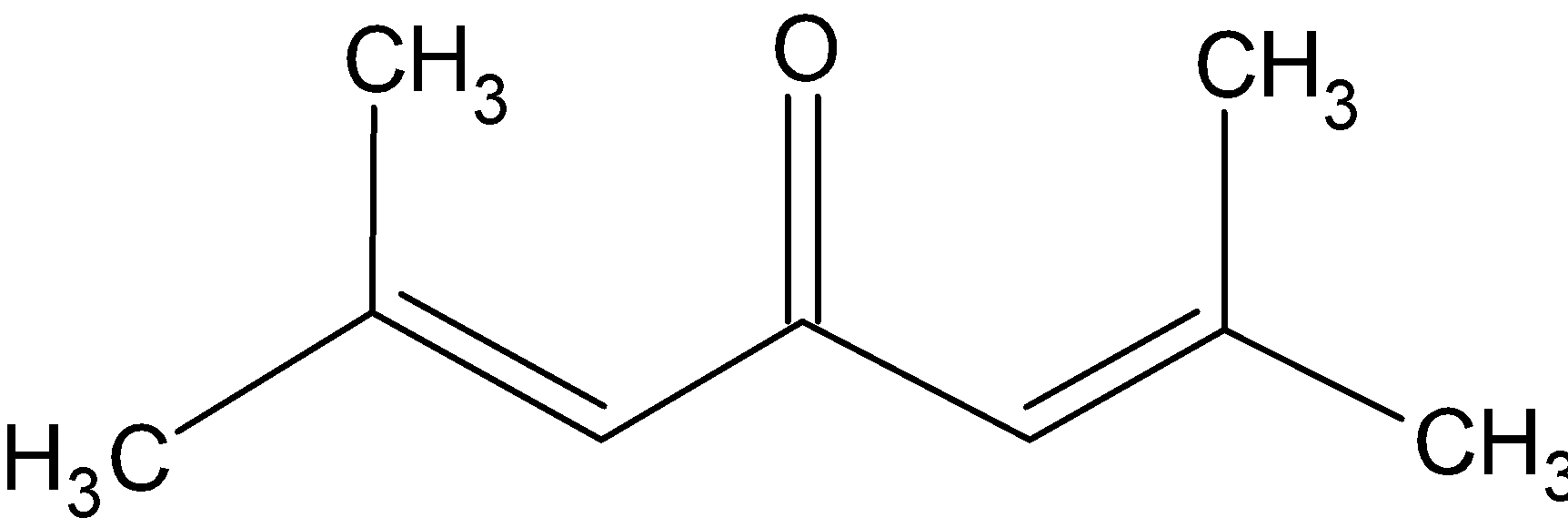
The molecular mass of the compound can be calculated now. It has 9 carbon atoms, one oxygen atom and 14 hydrogen atoms. So the molecular weight of phorone is $ = 9\left( {12} \right) + 1\left( {16} \right) + 14\left( 1 \right) = 138 $
Acetone is the common name for the compound propanone. Its molecular weight is 58.
The molecular mass of water molecules is 18.
So we can write a relation between the three masses as $ 3\left( {58} \right) - 2\left( {18} \right) = 138 $
So the molecular weight of phorone is 3 molecular weight of acetone – 2 molecular weight of water
The correct answer is B.
Note:
You can also answer this question if you know the process of formation of phorone. Phorone is prepared by the condensation of 3 acetone molecules. In the reaction, 2 molecules of water are lost and one molecule of phorone is formed. So the mass of the molecule can be written simple as
3 molecular weight of acetone – 2 molecular weight of water
Complete step by step solution:
Phorone is the common name for the compound diIsopropylidene acetone. It is a crystalline yellow coloured substance and it has an odour like that of germanium. Phorone is a ketone with double bonds attached on both of its alpha carbon atoms.
According to IUPAC nomenclature, the name of the compound phorone is 2, 6- Dimethyl 2, 5- heptadien- 4- one. The IUPAC nomenclature of organic compounds has set rules in such a way that each organic compound has a unique name.
Knowing the IUPAC name of phorone as 2, 6- Dimethyl 2, 5- heptadien- 4- one, we can draw its structure as

The molecular mass of the compound can be calculated now. It has 9 carbon atoms, one oxygen atom and 14 hydrogen atoms. So the molecular weight of phorone is $ = 9\left( {12} \right) + 1\left( {16} \right) + 14\left( 1 \right) = 138 $
Acetone is the common name for the compound propanone. Its molecular weight is 58.
The molecular mass of water molecules is 18.
So we can write a relation between the three masses as $ 3\left( {58} \right) - 2\left( {18} \right) = 138 $
So the molecular weight of phorone is 3 molecular weight of acetone – 2 molecular weight of water
The correct answer is B.
Note:
You can also answer this question if you know the process of formation of phorone. Phorone is prepared by the condensation of 3 acetone molecules. In the reaction, 2 molecules of water are lost and one molecule of phorone is formed. So the mass of the molecule can be written simple as
3 molecular weight of acetone – 2 molecular weight of water
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




