
What molecular structure is expected for iodine trichloride \[IC{{l}_{3}}\] molecule according to the VSEPR model?
A. T- shaped
B. See- saw
C. Trigonal planar
D. Linear
Answer
539.4k+ views
Hint: the full form of VSEPR is the valence shell electron pair repulsion theory. VSEPR theory states that the repulsion present between the two electrons is due to the Pauli exclusion principle. The founder of this theory was Ronald Nyholm and Ronald Gillespie. It is assumed that the molecule will take the shape in which the electronic repulsion in the valence shell will be minimized.
Complete step by step answer:
The valence shell electron pair repulsion theory states that the total number of the valence shell electron pair decides the shape of the molecule. In the polyatomic molecules one of the atoms will be the central atom. The valence shell is considered to be the sphere in shape and the distance between the electron pair is maximized. The strength of the repulsion between the lone pairs is strong and the weakest between the bond pairs.
The iodine trichloride is the inter halogenic compound which has 2 lone pairs and 3 bond pairs. The expected shape of iodine trichloride is T- shaped but the original shape of the iodine trichloride is trigonal bipyramidal. So the molecular shape is the following:
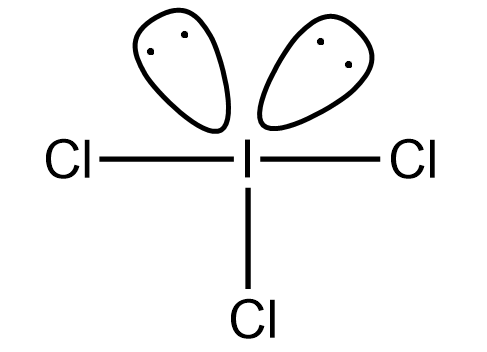
Hence option (A) is correct.
Note: this theory was not able to explain isoelectronic species. It was not able to determine the correct shape for the transition metals. It predicted that the halides of group 2 will have linear structure but actually it was bent in shape. The least electronegative atom will be the central atom.
Complete step by step answer:
The valence shell electron pair repulsion theory states that the total number of the valence shell electron pair decides the shape of the molecule. In the polyatomic molecules one of the atoms will be the central atom. The valence shell is considered to be the sphere in shape and the distance between the electron pair is maximized. The strength of the repulsion between the lone pairs is strong and the weakest between the bond pairs.
The iodine trichloride is the inter halogenic compound which has 2 lone pairs and 3 bond pairs. The expected shape of iodine trichloride is T- shaped but the original shape of the iodine trichloride is trigonal bipyramidal. So the molecular shape is the following:

Hence option (A) is correct.
Note: this theory was not able to explain isoelectronic species. It was not able to determine the correct shape for the transition metals. It predicted that the halides of group 2 will have linear structure but actually it was bent in shape. The least electronegative atom will be the central atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




