
What molecular structure is expected for iodine molecules according to the VSEPR model?
A.T-shaped
B. See-saw
C. Trigonal planar
D. Linear
Answer
594k+ views
Hint: We know that valence shell electron pair repulsion (VSEPR) theory is used to determine the geometry of molecules from the electron pairs surrounding the central atom.
Complete step by step answer:
First, we draw the structure of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule. We know that iodine is an element belonging to group 17 of the periodic table. Chlorine is also an element that belongs to the same group as iodine. The number of valence electrons of iodine is 7. Out of seven valence electrons, three electrons are shared with three chlorine atoms to form three ${\rm{I}} - {\rm{Cl}}$ bond and two pairs of lone pair electrons. So, the structure of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule is,
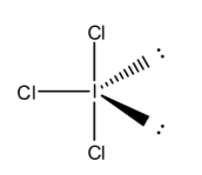
From the above Lewis structure of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule, we clearly see that the position of two lone pairs at the equatorial position in the trigonal bipyramidal geometry. The positions of lone pairs are$120^\circ $ apart, so they experience least repulsion compared to axial position. So, the shape of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule is T-shaped.
So, the correct answer is “Option A”.
Additional Information:
According to VSEPR theory, lone pair electrons repel each other more strongly than that of bond pair electrons. So, the decreasing order of repulsion is lp-lp>lp-bp>bp-bp.
So, repulsion between bond pair-bond pair is least and between lone pair-lone pair is highest.
Note:
Students might think that as Iodine has three bond pairs in ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$, its molecular shape is trigonal planar. But it is not correct. The two lone pairs also impact on the shape of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule. If only three bond pairs present in a compound, then its molecular shape is trigonal planar, such as, in case of ${\rm{B}}{{\rm{H}}_{\rm{3}}}$.
Complete step by step answer:
First, we draw the structure of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule. We know that iodine is an element belonging to group 17 of the periodic table. Chlorine is also an element that belongs to the same group as iodine. The number of valence electrons of iodine is 7. Out of seven valence electrons, three electrons are shared with three chlorine atoms to form three ${\rm{I}} - {\rm{Cl}}$ bond and two pairs of lone pair electrons. So, the structure of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule is,

From the above Lewis structure of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule, we clearly see that the position of two lone pairs at the equatorial position in the trigonal bipyramidal geometry. The positions of lone pairs are$120^\circ $ apart, so they experience least repulsion compared to axial position. So, the shape of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule is T-shaped.
So, the correct answer is “Option A”.
Additional Information:
According to VSEPR theory, lone pair electrons repel each other more strongly than that of bond pair electrons. So, the decreasing order of repulsion is lp-lp>lp-bp>bp-bp.
So, repulsion between bond pair-bond pair is least and between lone pair-lone pair is highest.
Note:
Students might think that as Iodine has three bond pairs in ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$, its molecular shape is trigonal planar. But it is not correct. The two lone pairs also impact on the shape of ${\rm{IC}}{{\rm{l}}_{\rm{3}}}$ molecule. If only three bond pairs present in a compound, then its molecular shape is trigonal planar, such as, in case of ${\rm{B}}{{\rm{H}}_{\rm{3}}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




