
Molecular shapes of $S{F_4}$ , $C{F_4}$ , $Xe{F_4}$ are:
A. The same with $2,0,1$ lone pairs of electrons respectively
B. The same with $1,1,1$ lone pairs of electrons respectively
C. The same with $0,1,2$ lone pairs of electrons respectively
D. Different with $0,1,2$ lone pairs of electrons respectively
Answer
546.3k+ views
Hint:According to question, we need to draw the geometries of the compounds given above. First we have to write the configuration or hybridisation of the central atom of the element. By which we get an idea about the bonds and lone pairs connected through it. According to VSEPR theory the geometry of any compound is seen on the basis of lone pairs and bond pairs around it which are arranged so as to decrease the electron pair repulsion.
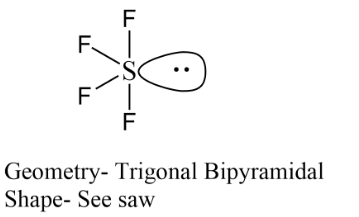
Complete step-by-step answer:Firstly we have sulphur tetrafluoride, in the formation of $S{F_4}$ the hybridisation of the central atom is $s{p^3}d$. It means five atoms or lone pairs are arranged around the central atom sulphur. As sulphur has four bonds with fluorine, leaving these four bonds we get only one lone pair of electrons thus its geometry can be shown as below. As a result, only $1$ lone pair of electrons is left with sulphur. Due to the $5$ lone pair of electrons, it should be form trigonal bipyramidal shaped structure but with two fluorine atoms occupy equatorial position and two occupy axial position, therefore its shape is see-saw.
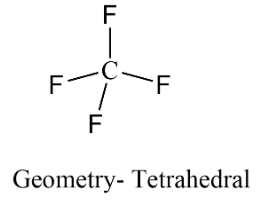
In the formation of $C{F_4}$ the hybridisation of the central atom is $s{p^3}$. So carbon has is connected with four fluorine atoms thus making a tetrahedral geometry. As all the valences are fulfilled by bon formation with fluorine therefore zero lone pair of electrons on carbon left and it forms a tetrahedral shaped structure.
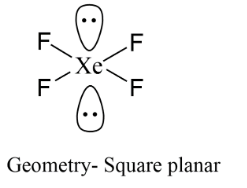
In the formation of $Xe{F_4}$ the hybridisation of the central atom is $s{p^3}{d^2}$ . It means six bonds are around xenon. We have four fluorine atoms in the formula and remaining are lone pairs thus we can arrange them so that there is minimum repulsion between bond pairs and lone pairs. Its structure should be octahedral, but because of the presence of two lone pairs of electrons which occupy axial positions, its shape is Square Planar.
So, the all three compounds have different molecular shapes with $0,1,2$ lone pairs respectively.
Hence, the correct option is (D.) different with $0,1,2$ lone pairs of electrons respectively.
Note:There is a difference between shape and geometry. In geometry we consider lone pair and bone pair while in shape we only consider bond pair. We can also get the molecular shape by drawing the Lewis Structure. And count the number of electron groups and determine them as lone pair or bond pair of electrons. Then name that group structure, looking at the positions of other atomic nuclei and determine the Molecular Structure.
Complete step-by-step answer:Firstly we have sulphur tetrafluoride, in the formation of $S{F_4}$ the hybridisation of the central atom is $s{p^3}d$. It means five atoms or lone pairs are arranged around the central atom sulphur. As sulphur has four bonds with fluorine, leaving these four bonds we get only one lone pair of electrons thus its geometry can be shown as below. As a result, only $1$ lone pair of electrons is left with sulphur. Due to the $5$ lone pair of electrons, it should be form trigonal bipyramidal shaped structure but with two fluorine atoms occupy equatorial position and two occupy axial position, therefore its shape is see-saw.

In the formation of $C{F_4}$ the hybridisation of the central atom is $s{p^3}$. So carbon has is connected with four fluorine atoms thus making a tetrahedral geometry. As all the valences are fulfilled by bon formation with fluorine therefore zero lone pair of electrons on carbon left and it forms a tetrahedral shaped structure.

In the formation of $Xe{F_4}$ the hybridisation of the central atom is $s{p^3}{d^2}$ . It means six bonds are around xenon. We have four fluorine atoms in the formula and remaining are lone pairs thus we can arrange them so that there is minimum repulsion between bond pairs and lone pairs. Its structure should be octahedral, but because of the presence of two lone pairs of electrons which occupy axial positions, its shape is Square Planar.

So, the all three compounds have different molecular shapes with $0,1,2$ lone pairs respectively.
Hence, the correct option is (D.) different with $0,1,2$ lone pairs of electrons respectively.
Note:There is a difference between shape and geometry. In geometry we consider lone pair and bone pair while in shape we only consider bond pair. We can also get the molecular shape by drawing the Lewis Structure. And count the number of electron groups and determine them as lone pair or bond pair of electrons. Then name that group structure, looking at the positions of other atomic nuclei and determine the Molecular Structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




