
What is the molecular shape of hydrogen sulphide molecule ${H_2}S$?
Answer
509.4k+ views
Hint: One of the most important theory which is used to predict the molecular shape and geometry of a molecule is valence shell electron pair repulsion theory i.e., VSEPR theory which is based on a concept that the lone pairs and bond pairs in a molecule will be arranged in such a manner that the repulsive forces are minimum.
Complete answer:
According to VSEPR theory,
1.In a polyatomic molecule i.e., a molecule which consists of three or more atoms, must have a central atom and all other atoms present in the molecule will form a bond with that central atom.
2.The total number of valence electrons plays a major role in deciding the shape of the molecule.
3.The lone pair of electrons on an atom has the tendency to arrange itself in such a way, that the repulsion is minimum.
4.If the central atom consists of both lone pairs as well as bond pairs, then the resulting shape of the molecule will be distorted.
5.The geometry of the molecule is decided by its steric number.
Therefore, for hydrogen sulphide molecule i.e., ${H_2}S$
Steric number $ = 4$
Corresponding hybridized state $ = s{p^3}$
Expected geometry is tetrahedral for the given case, but due to presence of two lone pair of electrons the geometry is distorted and the molecule forms a bent shape structure as follows:
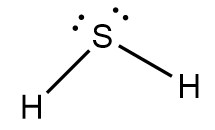
Hence, the molecular shape of hydrogen sulphide is bent.
Note:
It is important to note that the number of atoms, groups and lone pairs connected to the central atom together make up the steric number of the molecule. In simple words, steric number is the summation of the number of lone pairs on a central atom and number of atoms connected to it via a single bond. For example, in ${H_2}S$, there are two bond pairs and two lone pairs. Therefore, the steric number of ${H_2}S$ is four.
Complete answer:
According to VSEPR theory,
1.In a polyatomic molecule i.e., a molecule which consists of three or more atoms, must have a central atom and all other atoms present in the molecule will form a bond with that central atom.
2.The total number of valence electrons plays a major role in deciding the shape of the molecule.
3.The lone pair of electrons on an atom has the tendency to arrange itself in such a way, that the repulsion is minimum.
4.If the central atom consists of both lone pairs as well as bond pairs, then the resulting shape of the molecule will be distorted.
5.The geometry of the molecule is decided by its steric number.
Therefore, for hydrogen sulphide molecule i.e., ${H_2}S$
Steric number $ = 4$
Corresponding hybridized state $ = s{p^3}$
Expected geometry is tetrahedral for the given case, but due to presence of two lone pair of electrons the geometry is distorted and the molecule forms a bent shape structure as follows:

Hence, the molecular shape of hydrogen sulphide is bent.
Note:
It is important to note that the number of atoms, groups and lone pairs connected to the central atom together make up the steric number of the molecule. In simple words, steric number is the summation of the number of lone pairs on a central atom and number of atoms connected to it via a single bond. For example, in ${H_2}S$, there are two bond pairs and two lone pairs. Therefore, the steric number of ${H_2}S$ is four.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




