
What is the molecular geometry of $SbC{l_3}$ ?
Answer
517.2k+ views
Hint: We have to know that the molecular geometry is the three-dimensional plane of the iotas that establish a particle. It incorporates the overall state of the particle just as security lengths, security points, torsional points, and whatever other mathematical boundaries that decide the situation of every atom.
Complete answer:
We have to know, some steps used to find the shape of the molecule that has to be given,
To summarize there are four straightforward strides to apply the VSEPR hypothesis.
First, we have to draw the Lewis structure.
Then, check the quantity of electron gatherings and recognize them as bond sets of electron gatherings or solitary sets of electrons. Recollect electron bunches incorporate securities as well as solitary sets!
Now, name the electron-bunch calculation. (State whether it is straight, three-sided planar, tetrahedral, three-sided bipyramidal, or octahedral.)
Finally, taking a gander at the places of other nuclear cores around the focal points decides the sub-atomic math. (Perceive the number of solitary sets there are.)
Molecular geometry impacts a few properties of a substance including its reactivity, extremity, period of issue, shading, attraction, and organic movement. The points between bonds that a particle structures depend just feebly on the remainder of the atom, for example they can be perceived as around nearby and henceforth adaptable properties.
The Lewis structure of $SbC{l_3}$ has to be drawn below,
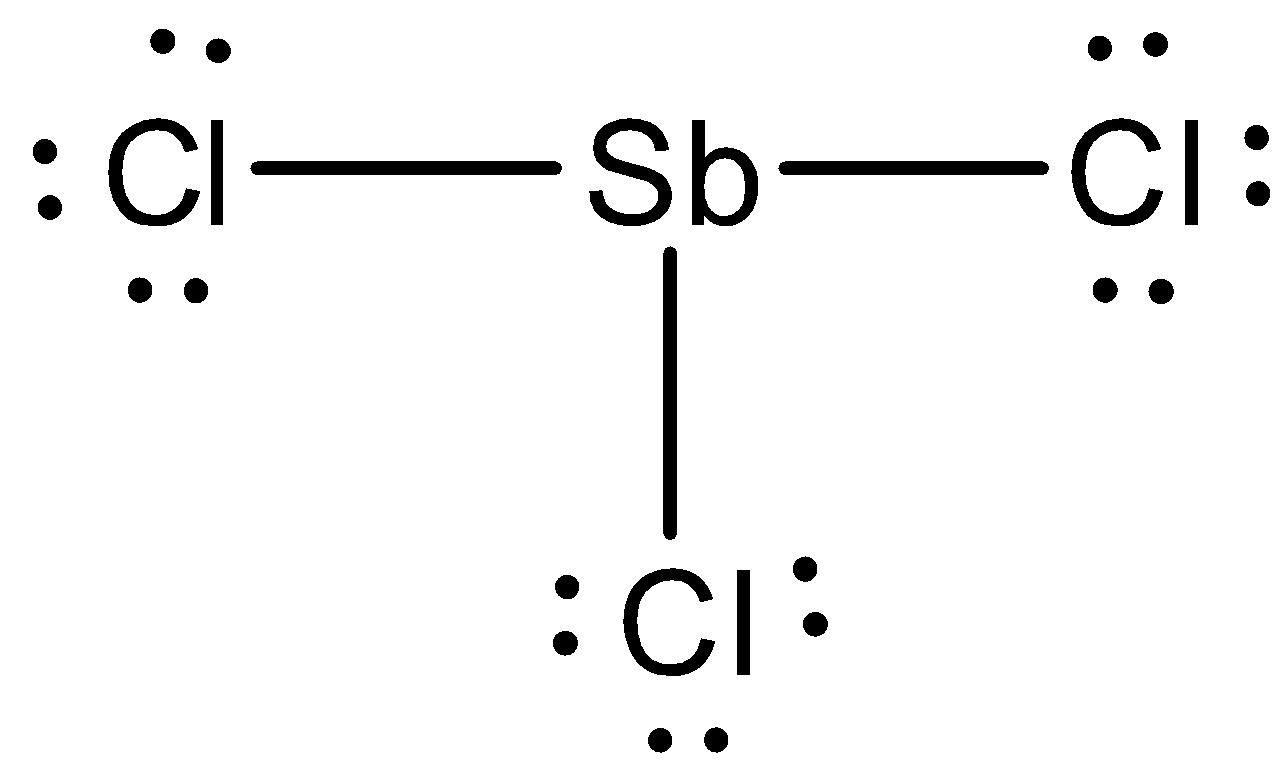
We have to find out the molecular geometry of $SbC{l_3}$ , around the focal tin particle, there are four electron sets, three are holding, and one non-holding. Therefore, the molecular geometry is trigonal pyramidal.
Note:
We have to know that, the reason for the VSEPR hypothesis is that electron sets situated in bonds and solitary sets repulse one another and will along these lines receive the math that places electron sets as far separated from one another as could really be expected.
Complete answer:
We have to know, some steps used to find the shape of the molecule that has to be given,
To summarize there are four straightforward strides to apply the VSEPR hypothesis.
First, we have to draw the Lewis structure.
Then, check the quantity of electron gatherings and recognize them as bond sets of electron gatherings or solitary sets of electrons. Recollect electron bunches incorporate securities as well as solitary sets!
Now, name the electron-bunch calculation. (State whether it is straight, three-sided planar, tetrahedral, three-sided bipyramidal, or octahedral.)
Finally, taking a gander at the places of other nuclear cores around the focal points decides the sub-atomic math. (Perceive the number of solitary sets there are.)
Molecular geometry impacts a few properties of a substance including its reactivity, extremity, period of issue, shading, attraction, and organic movement. The points between bonds that a particle structures depend just feebly on the remainder of the atom, for example they can be perceived as around nearby and henceforth adaptable properties.
The Lewis structure of $SbC{l_3}$ has to be drawn below,

We have to find out the molecular geometry of $SbC{l_3}$ , around the focal tin particle, there are four electron sets, three are holding, and one non-holding. Therefore, the molecular geometry is trigonal pyramidal.
Note:
We have to know that, the reason for the VSEPR hypothesis is that electron sets situated in bonds and solitary sets repulse one another and will along these lines receive the math that places electron sets as far separated from one another as could really be expected.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




