
What is the molecular geometry of $ NO_{3}^{-} $ ion?
Answer
515.1k+ views
Hint: The molecular geometry of an ion or molecule can be predicted using valence shell electron pair repulsion theory i.e., VSEPR theory. It is based on assumptions that the lone pair and bond pairs of an ion will be arranged in such a manner, that the force of repulsion is minimized.
Complete answer:
According to VSEPR theory, in a polyatomic molecule or ion i.e., a molecule which consists of more than three atoms, one of the atoms is termed as the central atom while other atoms or ions are bonded to it.
To decide the geometry of a molecule, following rules must be followed:
The central atom must be the least electronegative atom because it donates its electrons to show an electropositive character.
The summation of the number of lone pairs or atoms bonded to the central atom via a single bond is known as the steric number of the molecule. It is also known as the VSEPR number. The geometry and hybridization should be assigned according to the steric number of a molecule.
Hence, for $ NO_{3}^{-} $ ion, nitrogen will be the central atom because it is less electronegative than oxygen.
Steric number $ =3 $
Hybridization $ =s{{p}^{2}} $
Hence, the molecular geometry of $ NO_{3}^{-} $ is trigonal planar.
Note:
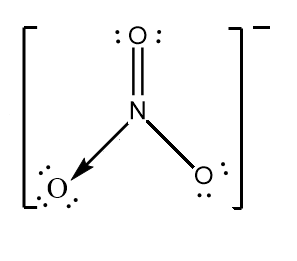
It is important to note that in $ NO_{3}^{-} $ ion, the nitrogen atom cannot form two double bonds with the oxygen atom because its maximum covalency is four. Hence, in the structure it is bonded with two oxygen atoms via double and single bond while forming a dative bond with the third oxygen atom.
Complete answer:
According to VSEPR theory, in a polyatomic molecule or ion i.e., a molecule which consists of more than three atoms, one of the atoms is termed as the central atom while other atoms or ions are bonded to it.
To decide the geometry of a molecule, following rules must be followed:
The central atom must be the least electronegative atom because it donates its electrons to show an electropositive character.
The summation of the number of lone pairs or atoms bonded to the central atom via a single bond is known as the steric number of the molecule. It is also known as the VSEPR number. The geometry and hybridization should be assigned according to the steric number of a molecule.
Hence, for $ NO_{3}^{-} $ ion, nitrogen will be the central atom because it is less electronegative than oxygen.
Steric number $ =3 $
Hybridization $ =s{{p}^{2}} $
Hence, the molecular geometry of $ NO_{3}^{-} $ is trigonal planar.

Note:
It is important to note that in $ NO_{3}^{-} $ ion, the nitrogen atom cannot form two double bonds with the oxygen atom because its maximum covalency is four. Hence, in the structure it is bonded with two oxygen atoms via double and single bond while forming a dative bond with the third oxygen atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




