
What is the molecular geometry of $ BC{l_3} $ ? Draw its VSEPR and Lewis structure.
Answer
537k+ views
Hint: VSEPR theory is basically used to predict the shape of molecules by using systematic steps. Whereas, lewis structure is also known as lewis dots, it is a representation of valence electrons in a given molecule.
Complete answer:
Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure.
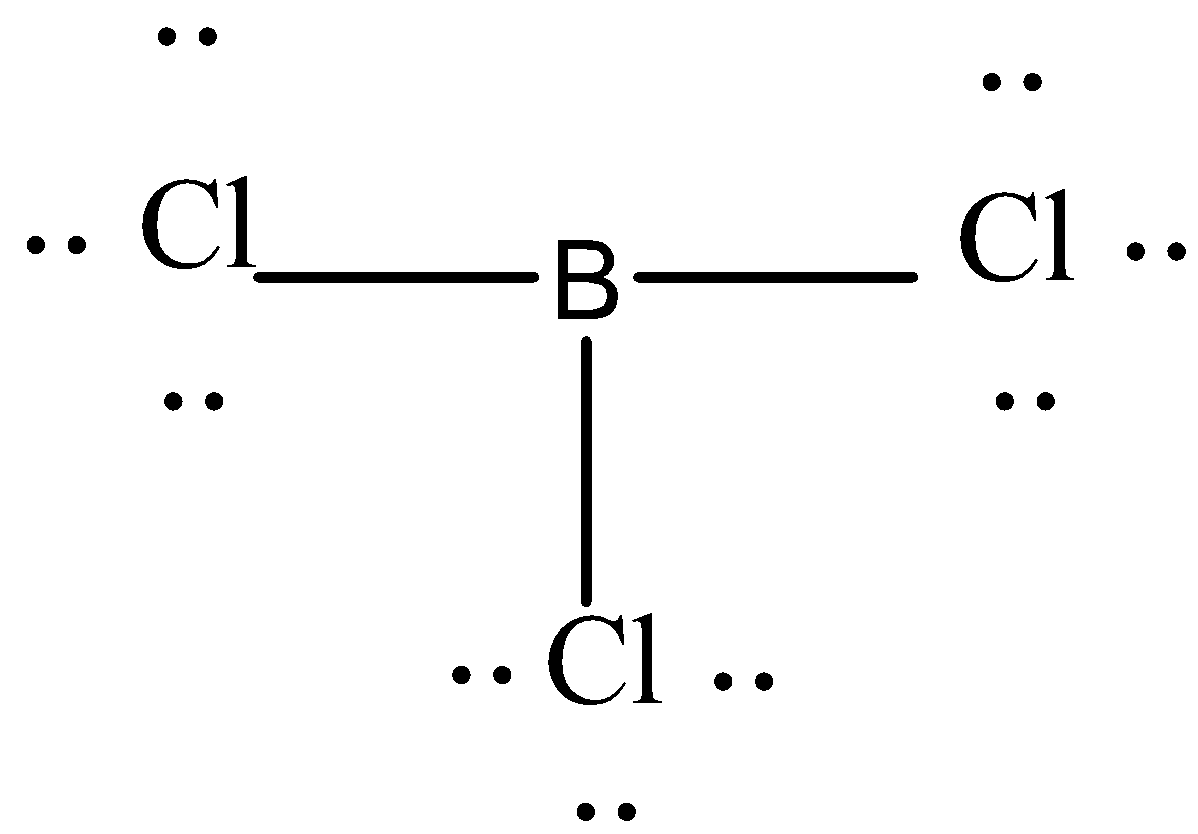
In this Lewis structure every chlorine has three lone pairs of electrons around it.
That is why it has $ s{p^2} $ hybridisation so the molecular geometry will be trigonal planar and the bond angle will be $ {120^o} $ .
So the VSEPR structure will look something like this:
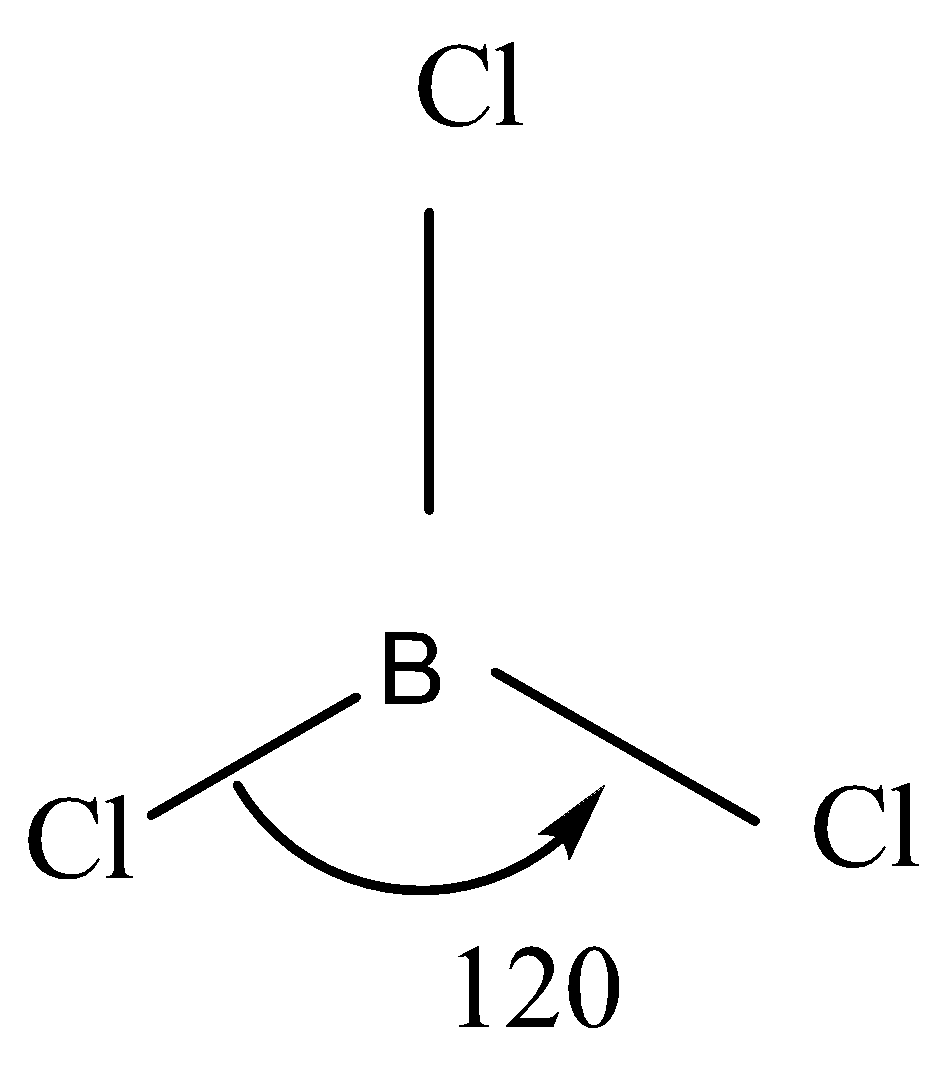
Note:
VSEPR is valence shell electron pair repulsion, it simply tells us that nonbonding and bonding electron pairs of the central atom in a molecule push each other away from each other in $ 3D $ space due to which molecules acquire their individual molecular shapes.
Complete answer:
Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure.

In this Lewis structure every chlorine has three lone pairs of electrons around it.
That is why it has $ s{p^2} $ hybridisation so the molecular geometry will be trigonal planar and the bond angle will be $ {120^o} $ .
So the VSEPR structure will look something like this:

Note:
VSEPR is valence shell electron pair repulsion, it simply tells us that nonbonding and bonding electron pairs of the central atom in a molecule push each other away from each other in $ 3D $ space due to which molecules acquire their individual molecular shapes.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




