
What is the molecular geometry of $ BC{l_3} $ ? Draw its VSEPR and Lewis structure.
Answer
528.3k+ views
Hint: VSEPR theory is basically used to predict the shape of molecules by using systematic steps. Whereas, lewis structure is also known as lewis dots, it is a representation of valence electrons in a given molecule.
Complete answer:
Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure.
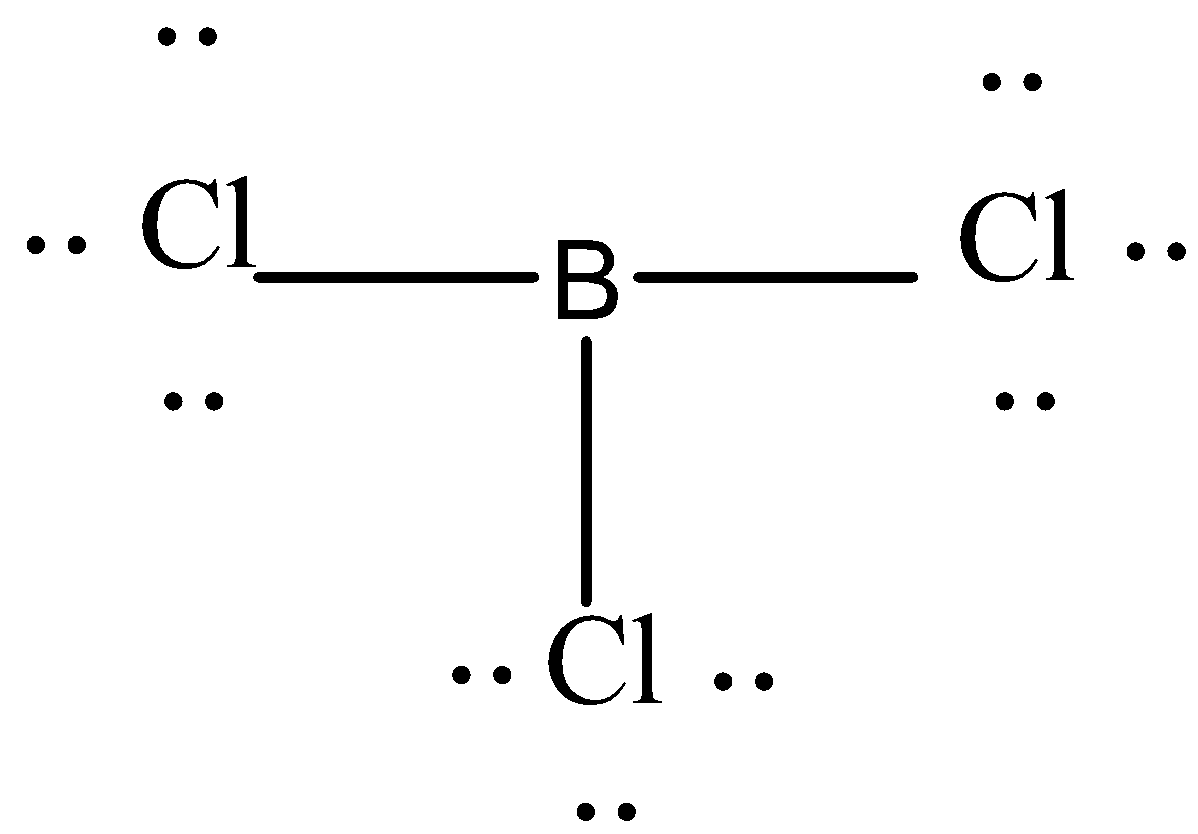
In this Lewis structure every chlorine has three lone pairs of electrons around it.
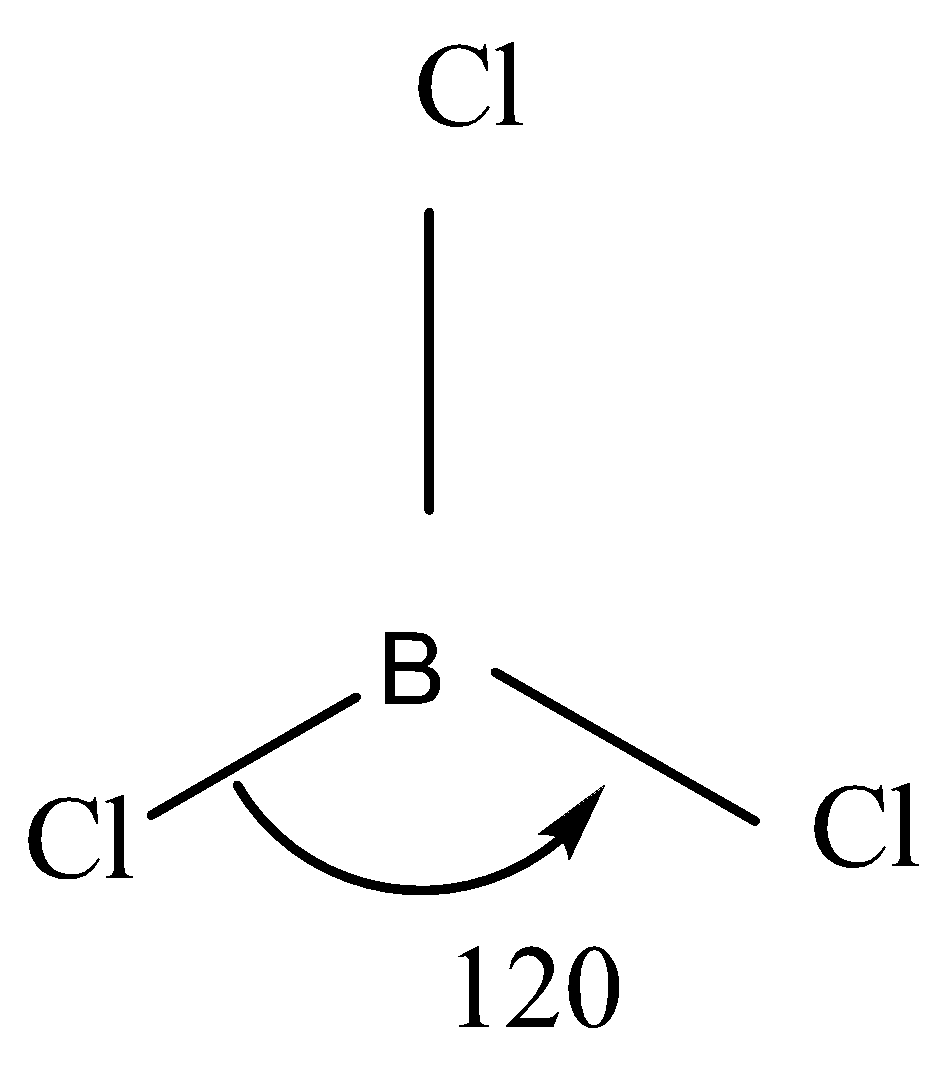
That is why it has $ s{p^2} $ hybridisation so the molecular geometry will be trigonal planar and the bond angle will be $ {120^o} $ .
So the VSEPR structure will look something like this:
Note:
VSEPR is valence shell electron pair repulsion, it simply tells us that nonbonding and bonding electron pairs of the central atom in a molecule push each other away from each other in $ 3D $ space due to which molecules acquire their individual molecular shapes.
Complete answer:
Let's first try to draw the Lewis structure of trichloride. It has three chlorine atoms and only one boron so boron will be a central atom in this molecular structure.

In this Lewis structure every chlorine has three lone pairs of electrons around it.
That is why it has $ s{p^2} $ hybridisation so the molecular geometry will be trigonal planar and the bond angle will be $ {120^o} $ .
So the VSEPR structure will look something like this:

Note:
VSEPR is valence shell electron pair repulsion, it simply tells us that nonbonding and bonding electron pairs of the central atom in a molecule push each other away from each other in $ 3D $ space due to which molecules acquire their individual molecular shapes.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




