
What is the molecular formula of pyrogallol?
(A) ${{C}_{6}}{{H}_{4}}{{\left( OH \right)}_{2}}$
(B) ${{C}_{6}}{{H}_{5}}{{\left( OH \right)}_{3}}$
(C) ${{C}_{6}}{{H}_{3}}{{\left( OH \right)}_{3}}$
(D) ${{C}_{6}}{{H}_{5}}OH$
Answer
581.7k+ views
Hint: The empirical formula of pyrogallol is ${{C}_{6}}{{H}_{6}}{{O}_{3}}$.
The IUPAC name and the structure can be different when it is considered in spatial form.
Complete step by step solution:
Pyrogallol is also called as pyrogallic acid and 1,2,3- trihydroxybenzene. It is an organic compound belonging to the phenol family, used as a photographic film developer and in preparation of other chemicals.
It is reported to be the best phenolic antioxidant for biodiesel.
It is a white, water-soluble solid although samples are typically brownish because of its sensitivity towards oxygen. It is one of the three isomeric benzenetriols. Organic solvents dissolve pyrogallol. But has low solubility in oils.
Pyrogallic acid is odourless white to grey. It is a weak acid crystalline compound chiefly used as a developer in photography.
Pyrogallol was obtained from gallic acid which is obtained from barks of various trees. Gallic acid is converted to pyrogallol under pressure by heating with water. Presently, gallic acid is obtained from tannin. Heating includes decarboxylation. Many alternative routes of pyrogallol production have been practised, as tannin is expensive.
In alkaline solution, it absorbs oxygen from the air and turns brown from colourless solution. It can be used to calculate the amount of oxygen in the air (Orsat analysis). As the metabolic rate is high and respiration requires large amounts of oxygen to fulfil the energy needs, alkaline pyrogallol is used in seed germination.
Pyrogallol has antiseptic properties and is used for hair dyeing. But this practice is declining day by day because of its toxicity.
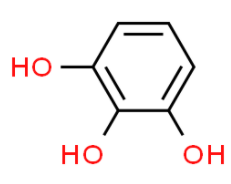
The formula of pyrogallol is ${{C}_{6}}{{H}_{3}}{{\left( OH \right)}_{3}}$.
Therefore, option (C) is correct.
Note: The molecular formula of pyrogallol is ${{C}_{6}}{{H}_{6}}{{O}_{3}}$ which can be considered as ${{C}_{6}}{{H}_{3}}{{\left( OH \right)}_{3}}$ according to the other name (IUPAC name) of pyrogallol as 1,2,3- trihydroxybenzene. Also, according to the common molecular formula, two options i.e. (A) and (B) can be ignored from the prior step.
The IUPAC name and the structure can be different when it is considered in spatial form.
Complete step by step solution:
Pyrogallol is also called as pyrogallic acid and 1,2,3- trihydroxybenzene. It is an organic compound belonging to the phenol family, used as a photographic film developer and in preparation of other chemicals.
It is reported to be the best phenolic antioxidant for biodiesel.
It is a white, water-soluble solid although samples are typically brownish because of its sensitivity towards oxygen. It is one of the three isomeric benzenetriols. Organic solvents dissolve pyrogallol. But has low solubility in oils.
Pyrogallic acid is odourless white to grey. It is a weak acid crystalline compound chiefly used as a developer in photography.
Pyrogallol was obtained from gallic acid which is obtained from barks of various trees. Gallic acid is converted to pyrogallol under pressure by heating with water. Presently, gallic acid is obtained from tannin. Heating includes decarboxylation. Many alternative routes of pyrogallol production have been practised, as tannin is expensive.
In alkaline solution, it absorbs oxygen from the air and turns brown from colourless solution. It can be used to calculate the amount of oxygen in the air (Orsat analysis). As the metabolic rate is high and respiration requires large amounts of oxygen to fulfil the energy needs, alkaline pyrogallol is used in seed germination.
Pyrogallol has antiseptic properties and is used for hair dyeing. But this practice is declining day by day because of its toxicity.

The formula of pyrogallol is ${{C}_{6}}{{H}_{3}}{{\left( OH \right)}_{3}}$.
Therefore, option (C) is correct.
Note: The molecular formula of pyrogallol is ${{C}_{6}}{{H}_{6}}{{O}_{3}}$ which can be considered as ${{C}_{6}}{{H}_{3}}{{\left( OH \right)}_{3}}$ according to the other name (IUPAC name) of pyrogallol as 1,2,3- trihydroxybenzene. Also, according to the common molecular formula, two options i.e. (A) and (B) can be ignored from the prior step.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




