
What is the molecular formula of ethanoic acid?
Answer
524.7k+ views
Hint :Ethanoic acid is a carboxylic acid with a methyl group and a carboxyl functional group added. Ethanoic acid is the systematic IUPAC name for acetic acid. Glacial acetic acid is an acetic acid solution that has not been dissolved.
Complete Step By Step Answer:
In the solid-state of acetic acid, a chain of molecules can be seen, with individual molecules bound by hydrogen bonds. When ethanoic acid is present in a dilute solution, the dimers can be detected also in the liquid form. Solvents that foster hydrogen bonding have a negative impact on these dimers.
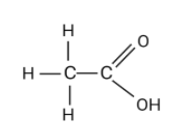
Now, to construct the molecular structure we will look into the naming nomenclature. There is an “eth” in ethanoic acid , this indicates that there are $2$carbon atoms in the compound. Now, One of these carbon atoms is attached with a carboxylic acid group as the compound name ends with “oic acid”. Hence, this carbon will be attached to double bond $O$ and single bond $OH$.
Therefore, the molecular formula for ethanoic acid is $C{H_3}COOH$.
Note :
Because of its antibacterial properties, acetic acid is used as an antiseptic. Ethanoic acid is used in the production of rayon fiber. Acetic acid has been used to cure tumors in the past by injecting it directly into the tumor. Since it is a major component of vinegar, it is used to pickle a variety of vegetables. Ethanoic acid is used in the manufacturing of rubber.
Complete Step By Step Answer:
In the solid-state of acetic acid, a chain of molecules can be seen, with individual molecules bound by hydrogen bonds. When ethanoic acid is present in a dilute solution, the dimers can be detected also in the liquid form. Solvents that foster hydrogen bonding have a negative impact on these dimers.
Now, to construct the molecular structure we will look into the naming nomenclature. There is an “eth” in ethanoic acid , this indicates that there are $2$carbon atoms in the compound. Now, One of these carbon atoms is attached with a carboxylic acid group as the compound name ends with “oic acid”. Hence, this carbon will be attached to double bond $O$ and single bond $OH$.
Therefore, the molecular formula for ethanoic acid is $C{H_3}COOH$.

Note :
Because of its antibacterial properties, acetic acid is used as an antiseptic. Ethanoic acid is used in the production of rayon fiber. Acetic acid has been used to cure tumors in the past by injecting it directly into the tumor. Since it is a major component of vinegar, it is used to pickle a variety of vegetables. Ethanoic acid is used in the manufacturing of rubber.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




