
What is the molecular formula of chlorate?
Answer
523.2k+ views
Hint: From the name, we can infer that it contains chlorine and oxygen
Chlorine can be represented by $Cl$
Oxygen can be represented by $O$
When the chlorate metal decomposes, chlorine and oxygen are released
Complete answer:
The chlorate includes combination of chlorine and oxygen
Chlorate is a monovalent inorganic anion
Chlorate is formed by the deprotonation of \[HCl{{O}_{3}}\]
Chlorate is a conjugate base of chloric acid
Chlorate can also be obtained due to the Oxoanion of Chlorine
Chlorate is non-combustible, soluble in water
Chlorate exist in the form of \[Cl{{O}^{3-}}\]
Chlorate is found by the natural breakdown of compounds either by sunlight or water
Chlorate formation depends on the composition of chlorine-di-oxide and their pH
Long term reaction of Chlorine with ignition material can result in explosion
Chlorate has major applications in explosives and pesticide
Chlorate is capable of reduction reactions
The molar mass of chlorate is 83.451
It exists in Trigonal pyramidal shape
Chlorate can also be formed by other method
Chlorate formation can also by the addition of hypochlorous acid and chlorite ion
The reaction between hypochlorous acid and chlorite ion leads to the formation of proton, chlorate and chloride ion
$HClO+Cl{{O}_{2}}^{-}\to C{{l}^{-}}+Cl{{O}_{3}}^{-}+{{H}^{+}}$ $HClO+Cl{{O}_{2}}^{-}\to C{{l}^{-}}+Cl{{O}_{3}}^{-}+{{H}^{+}}$
The structure of chlorate is
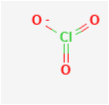
Note:
Chlorate is a combination of chlorine and oxygen.
Chlorate can also be formed by the reaction between hypochlorous acid and chlorite ion
Some of the applications of chlorate such as sodium chlorate has been banned as it can cause explosions
Chlorine can be represented by $Cl$
Oxygen can be represented by $O$
When the chlorate metal decomposes, chlorine and oxygen are released
Complete answer:
The chlorate includes combination of chlorine and oxygen
Chlorate is a monovalent inorganic anion
Chlorate is formed by the deprotonation of \[HCl{{O}_{3}}\]
Chlorate is a conjugate base of chloric acid
Chlorate can also be obtained due to the Oxoanion of Chlorine
Chlorate is non-combustible, soluble in water
Chlorate exist in the form of \[Cl{{O}^{3-}}\]
Chlorate is found by the natural breakdown of compounds either by sunlight or water
Chlorate formation depends on the composition of chlorine-di-oxide and their pH
Long term reaction of Chlorine with ignition material can result in explosion
Chlorate has major applications in explosives and pesticide
Chlorate is capable of reduction reactions
The molar mass of chlorate is 83.451
It exists in Trigonal pyramidal shape
Chlorate can also be formed by other method
Chlorate formation can also by the addition of hypochlorous acid and chlorite ion
The reaction between hypochlorous acid and chlorite ion leads to the formation of proton, chlorate and chloride ion
$HClO+Cl{{O}_{2}}^{-}\to C{{l}^{-}}+Cl{{O}_{3}}^{-}+{{H}^{+}}$ $HClO+Cl{{O}_{2}}^{-}\to C{{l}^{-}}+Cl{{O}_{3}}^{-}+{{H}^{+}}$
The structure of chlorate is

Note:
Chlorate is a combination of chlorine and oxygen.
Chlorate can also be formed by the reaction between hypochlorous acid and chlorite ion
Some of the applications of chlorate such as sodium chlorate has been banned as it can cause explosions
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




