
$Mn{O_2}$is fused with$KOH$ in the presence of air, a colored compound is formed, the product and its color is:
A. ${K_2}Mn{O_4},$dark green
B. $KMn{O_4}$, purple
C. $M{n_2}{O_3}$, brown
D. $M{n_3}{O_4}$,black
Answer
579k+ views
Hint: Redox reactions are one of those reactions in which oxidation and Reduction takes place simultaneously.
Increase in oxidation Number during reaction is known as oxidation.
Decrease in Reduction Number during ration is called Reduction.
During Redox reaction increase in oxidation number and decrease in oxidation number takes place simultaneously.
Complete step by step answer:
Let us discuss a given reaction $Mn{O_2}$ fused with$KOH$ in presence of air A green colored${K_2}Mn{O_4}$ is obtained.
$2Mn{O_2} + 4KOH + {O_2} \to 2{K_2}Mn{O_4} + 2{H_2}O$
Manages Potassium Potassium
Dioxide hydroxide magnate
Therefore, from the above explanation the correct option is (A) ${K_2}Mn{O_4},$ dark green.
This is the first step of preparation of potassium permanganate $\left[ {KMn{O_4}} \right]$.
In this reaction $2Mn{O_2}$ Oxidizes to ${K_2}Mn{O_4}$.
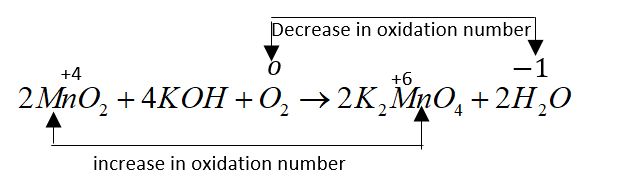
The fused mass obtained in the reaction contains ${K_2}Mn{O_4}$. It is Heated with water and men convert it into $KMn{O_4} $either by oxidation or by electrolysis.
Oxidation of ${K_2}Mn{O_4}$ to $KMn{O_4}$ carried out by ${H_2}S{O_4}$ or $C{l_2}$ or $C{O_2}$ thorough solution.
$3K\mathop {Mn{O_4}}\limits^{ + 6} + 2{H_2}S{O_4} \to 2{K_2}S{O_4} + 2K{\mathop {MnO}\limits^{ + 7} _4} + 2{H_2}O + \mathop {Mn{O_2}}\limits^{ + 4} $
$2{K_2}Mn{O_4} + C{l_2} \to 2KMn{O_4} + 2KCl$
First reaction is disproportion reaction in which ${K_2}Mn{O_4}$ Oxidizes to $KMn{O_4}$ and reduces to $Mn{O_2}$ In electrolytic oxidation magnate solution is electrolytic. between ion electrodes.
At $Ca$ mode $2{H^ + } + 2{e^ - } \to {H_2} \uparrow $ Reduction
The oxygen evolved at anode converts manganate to permanganate:
$2{K_2}Mn{O_4} + {H_2}O + \left[ O \right] \to 2KMn{O_4} + 2KOH.$
Note:
Potassium permanganate$\left[ {KMn{O_4}} \right]$ is. An oxidizing agent. It is used in the industry and laboratory.
It is used as Baeyer’s reagent for defecting unsaturation in organic compound
Increase in oxidation Number during reaction is known as oxidation.
Decrease in Reduction Number during ration is called Reduction.
During Redox reaction increase in oxidation number and decrease in oxidation number takes place simultaneously.
Complete step by step answer:
Let us discuss a given reaction $Mn{O_2}$ fused with$KOH$ in presence of air A green colored${K_2}Mn{O_4}$ is obtained.
$2Mn{O_2} + 4KOH + {O_2} \to 2{K_2}Mn{O_4} + 2{H_2}O$
Manages Potassium Potassium
Dioxide hydroxide magnate
Therefore, from the above explanation the correct option is (A) ${K_2}Mn{O_4},$ dark green.
This is the first step of preparation of potassium permanganate $\left[ {KMn{O_4}} \right]$.
In this reaction $2Mn{O_2}$ Oxidizes to ${K_2}Mn{O_4}$.
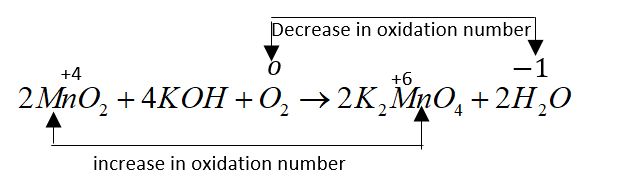
The fused mass obtained in the reaction contains ${K_2}Mn{O_4}$. It is Heated with water and men convert it into $KMn{O_4} $either by oxidation or by electrolysis.
Oxidation of ${K_2}Mn{O_4}$ to $KMn{O_4}$ carried out by ${H_2}S{O_4}$ or $C{l_2}$ or $C{O_2}$ thorough solution.
$3K\mathop {Mn{O_4}}\limits^{ + 6} + 2{H_2}S{O_4} \to 2{K_2}S{O_4} + 2K{\mathop {MnO}\limits^{ + 7} _4} + 2{H_2}O + \mathop {Mn{O_2}}\limits^{ + 4} $
$2{K_2}Mn{O_4} + C{l_2} \to 2KMn{O_4} + 2KCl$
First reaction is disproportion reaction in which ${K_2}Mn{O_4}$ Oxidizes to $KMn{O_4}$ and reduces to $Mn{O_2}$ In electrolytic oxidation magnate solution is electrolytic. between ion electrodes.
At $Ca$ mode $2{H^ + } + 2{e^ - } \to {H_2} \uparrow $ Reduction
The oxygen evolved at anode converts manganate to permanganate:
$2{K_2}Mn{O_4} + {H_2}O + \left[ O \right] \to 2KMn{O_4} + 2KOH.$
Note:
Potassium permanganate$\left[ {KMn{O_4}} \right]$ is. An oxidizing agent. It is used in the industry and laboratory.
It is used as Baeyer’s reagent for defecting unsaturation in organic compound
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




