
Michael addition is a conjugate addition i.e, $ 1-4 $ addition. The substrates of the reaction that are involved are the compounds like $ \alpha ,\beta - $ unsaturated carbonyl compounds, esters, cyanides, quinones and $ \alpha ,\beta - $ unsaturated nitro compounds. The reagent must have at least one acidic hydrogen and must be converted into electrophile in the presence of the bases like $ OH,OR $ , secondary amine etc.
In the following reaction, the product formed is: $ C{{H}_{2}}=CHCOC{{H}_{3}}+C{{H}_{3}}C{{H}_{2}}N{{O}_{2}}\xrightarrow[CHC{{l}_{3}}]{{{\left[ {{\left( C{{H}_{3}} \right)}_{2}}CH \right]}_{2}}NH} $
(A)
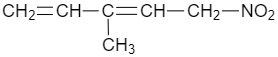
(B)
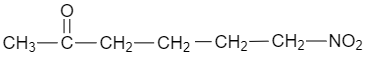
(C)
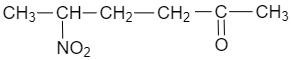
(D)
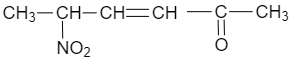
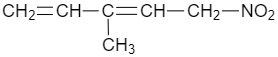
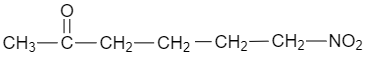
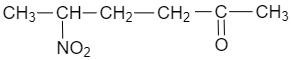
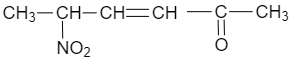
Answer
550.8k+ views
Hint :Nucleophiles act as a good Michael donor as they have very good substituent groups such as acyl groups and cyano groups. This Michael donor makes the methylene hydrogen acidic and results in the formation of carbanion when it reacts with a base.
Complete step by step solution:
In Michael's addition reaction, with the help of base, alpha hydrogen of nitro ethane is removed. Here, the alpha carbon acts as a nucleophile that attacks on $ C-4 $ carbon of but $ -3- $ enone, it results in $ 1,4- $ addition that gives $ 5- $ nitro hexan $ -2- $ one as a product.
Mechanism
In the first step, the nitro compound is attacked by the base. The alpha hydrogen of the nitro compound is deprotonated by the base and results in the formation of a carbanion.
The carbanion becomes stable due to electron withdrawing groups.. Now the enolate ion and Michael acceptor react with each other and form a carbon- carbon bond.
Now, the compound formed by $ 1,4 $ addition of the enolate on $ \alpha ,\beta - $ unsaturated carbonyl compounds gets protonated
Let us see the mechanism:
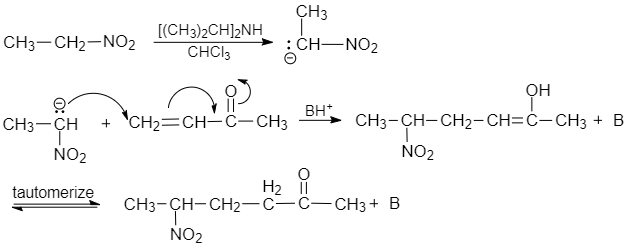
Let us see the reaction
$ C{{H}_{2}}=CHCOC{{H}_{3}}+C{{H}_{3}}C{{H}_{2}}N{{O}_{2}}\xrightarrow[CHC{{l}_{3}}]{{{\left[ {{\left( C{{H}_{3}} \right)}_{2}}CH \right]}_{2}}NH}C{{H}_{3}}CH(N{{O}_{2}})C{{H}_{2}}C{{H}_{2}}COC{{H}_{3}} $
Therefore, the correct option is C.
Note:
Nucleophiles are electron rich species that have the ability to donate electron pairs to an electrophile so that they form a chemical bond. Any compound that has a lone pair and pi bond containing two electrons has the ability to behave like a nucleophile.
Nucleophiles are referred to as Michael donors. They are generally electron withdrawing. Substituent groups on activated unsaturated compounds are Michael acceptors. Some examples of Michael acceptors are ketone groups and nitro groups.
Complete step by step solution:
In Michael's addition reaction, with the help of base, alpha hydrogen of nitro ethane is removed. Here, the alpha carbon acts as a nucleophile that attacks on $ C-4 $ carbon of but $ -3- $ enone, it results in $ 1,4- $ addition that gives $ 5- $ nitro hexan $ -2- $ one as a product.
Mechanism
In the first step, the nitro compound is attacked by the base. The alpha hydrogen of the nitro compound is deprotonated by the base and results in the formation of a carbanion.
The carbanion becomes stable due to electron withdrawing groups.. Now the enolate ion and Michael acceptor react with each other and form a carbon- carbon bond.
Now, the compound formed by $ 1,4 $ addition of the enolate on $ \alpha ,\beta - $ unsaturated carbonyl compounds gets protonated
Let us see the mechanism:

Let us see the reaction
$ C{{H}_{2}}=CHCOC{{H}_{3}}+C{{H}_{3}}C{{H}_{2}}N{{O}_{2}}\xrightarrow[CHC{{l}_{3}}]{{{\left[ {{\left( C{{H}_{3}} \right)}_{2}}CH \right]}_{2}}NH}C{{H}_{3}}CH(N{{O}_{2}})C{{H}_{2}}C{{H}_{2}}COC{{H}_{3}} $
Therefore, the correct option is C.
Note:
Nucleophiles are electron rich species that have the ability to donate electron pairs to an electrophile so that they form a chemical bond. Any compound that has a lone pair and pi bond containing two electrons has the ability to behave like a nucleophile.
Nucleophiles are referred to as Michael donors. They are generally electron withdrawing. Substituent groups on activated unsaturated compounds are Michael acceptors. Some examples of Michael acceptors are ketone groups and nitro groups.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




