
Methyl amine reacts with acetyl chloride to form:
(a) $C{{H}_{3}}N{{H}_{2}}$
(b) $C{{H}_{3}}NHNa$
(c) $C{{H}_{3}}NHCOC{{H}_{3}}$
(d) ${{\left( C{{H}_{3}} \right)}_{2}}NCOC{{H}_{3}}$
Answer
539.7k+ views
Hint:Amine group is one of the functional groups which has the formulae $-N{{H}_{2}}$ and considered as the derivative of ammonia. The acetyl chloride has a formulae, $C{{H}_{3}}(CO)-Cl$.The interchange of atoms in the reaction is only happening with the atoms attached to N in the amine group.
Complete step-by-step answer:So solve the question we should know the functional groups involved in the compounds and what change is happening between the molecules to predict the final product.
So let us first discuss the functional groups in each compound and its nature.
First let us discuss methyl amine. So from the term methyl we know that a $-C{{H}_{3}}$ group is present and the functional group is amine i.e. $-N{{H}_{2}}$group. Hence the methyl amine has the formulae $C{{H}_{3}}-N{{H}_{2}}$. So methyl amine is a primary amine. We could define amines and the derivatives of ammonia, because by replacing a hydrogen atom from ammonia atom with alkyl groups we could yield primary amine, secondary amine, tertiary amine etc. And as the ammonia the amines are also basic in nature.
Now let us discuss acetyl chloride. Acetyl chloride is also called ethanoyl chloride. So from the term ethanoyl it is clear that there are two carbon in the molecule and one is the carbonyl group. And the term chloride will give an idea that chloride is also present in the molecule. Acetyl chloride is a simplest acyl halide.
Now let us tale about the reaction In the reaction the acetyl chloride reacts with methyl amine and the chlorine atom is remove from the acetyl molecule and the amine gets attached to acetyl group by replacing H with an acetyl group and the H from the amine group and Cl from the acetyl chloride forms HCl.
The reaction is as follows:
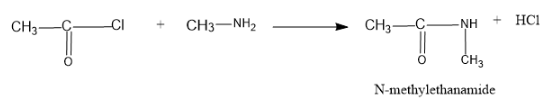
Hence the product formed is N-methyl ethanamide.
The correct answer for the question is option (c), which is $C{{H}_{3}}NHCOC{{H}_{3}}$.
Note:The acetyl chloride is derived from acetic acid.
And we know that the like ammonia, amines are also basic in nature, during the formation of N-methyl ethanamide, HCl is also formed, H from amine methyl amine and Cl from the acetyl chloride, the methyl amine if present in excess in the reaction the HCl formed will react with methyl amine and form a salt and the salt is methyl ammonium chloride and the reaction is as follows:
\[C{{H}_{3}}-N{{H}_{2}}+HCl\to C{{H}_{3}}-N{{H}_{3}}^{+}C{{l}^{-}}\]
Complete step-by-step answer:So solve the question we should know the functional groups involved in the compounds and what change is happening between the molecules to predict the final product.
So let us first discuss the functional groups in each compound and its nature.
First let us discuss methyl amine. So from the term methyl we know that a $-C{{H}_{3}}$ group is present and the functional group is amine i.e. $-N{{H}_{2}}$group. Hence the methyl amine has the formulae $C{{H}_{3}}-N{{H}_{2}}$. So methyl amine is a primary amine. We could define amines and the derivatives of ammonia, because by replacing a hydrogen atom from ammonia atom with alkyl groups we could yield primary amine, secondary amine, tertiary amine etc. And as the ammonia the amines are also basic in nature.
Now let us discuss acetyl chloride. Acetyl chloride is also called ethanoyl chloride. So from the term ethanoyl it is clear that there are two carbon in the molecule and one is the carbonyl group. And the term chloride will give an idea that chloride is also present in the molecule. Acetyl chloride is a simplest acyl halide.
Now let us tale about the reaction In the reaction the acetyl chloride reacts with methyl amine and the chlorine atom is remove from the acetyl molecule and the amine gets attached to acetyl group by replacing H with an acetyl group and the H from the amine group and Cl from the acetyl chloride forms HCl.
The reaction is as follows:

Hence the product formed is N-methyl ethanamide.
The correct answer for the question is option (c), which is $C{{H}_{3}}NHCOC{{H}_{3}}$.
Note:The acetyl chloride is derived from acetic acid.
And we know that the like ammonia, amines are also basic in nature, during the formation of N-methyl ethanamide, HCl is also formed, H from amine methyl amine and Cl from the acetyl chloride, the methyl amine if present in excess in the reaction the HCl formed will react with methyl amine and form a salt and the salt is methyl ammonium chloride and the reaction is as follows:
\[C{{H}_{3}}-N{{H}_{2}}+HCl\to C{{H}_{3}}-N{{H}_{3}}^{+}C{{l}^{-}}\]
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




