
Methyl acetylene is also known as:
a.) Butyne
b.) Propyne
c.) Ethyne
d.) Pentyne
Answer
590.1k+ views
Hint: The structure of acetylene molecule is as follows.
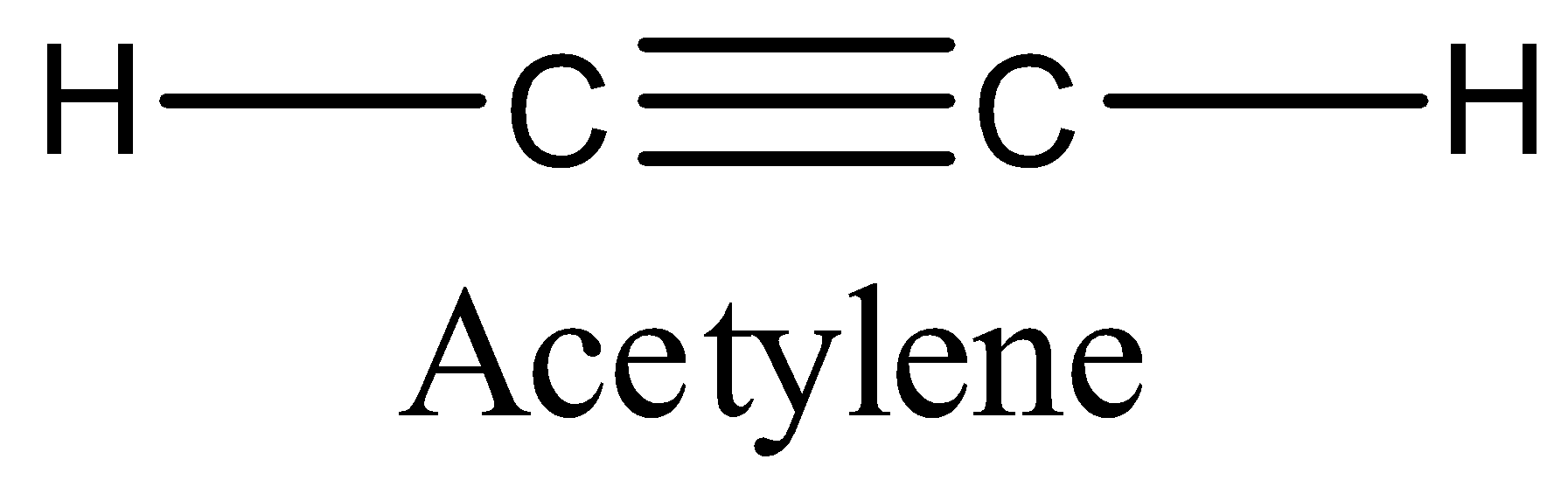
Acetylene contains two hydrogens, two carbons and one triple bond between two carbons. The molecular formula of acetylene is\[{{C}_{2}}{{H}_{2}}\].
Complete step by step answer:
In the question they asked the structure of the methyl acetylene.
The structure of methyl acetylene contains one more methyl group in addition to the structure of acetylene.
The structure of the methyl acetylene is as follows.
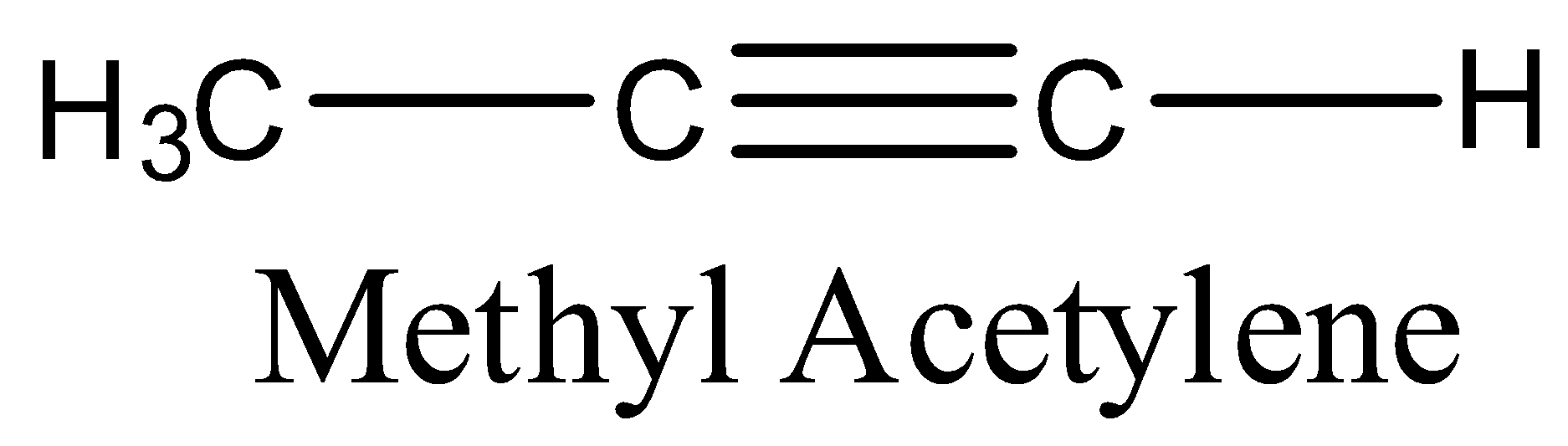
Methyl Acetylene contains four hydrogens, three carbons, one triple bond between two carbons and one single bond between two carbons.
The molecular formula of methyl acetylene is\[{{C}_{3}}{{H}_{4}}\].
Methyl acetylene is also called as propyne because it contains three carbons and one triple bond (alkyne).
Methyl acetylene is also called as Prop-1-yne (IUPAC name) because the triple is present at first carbon.
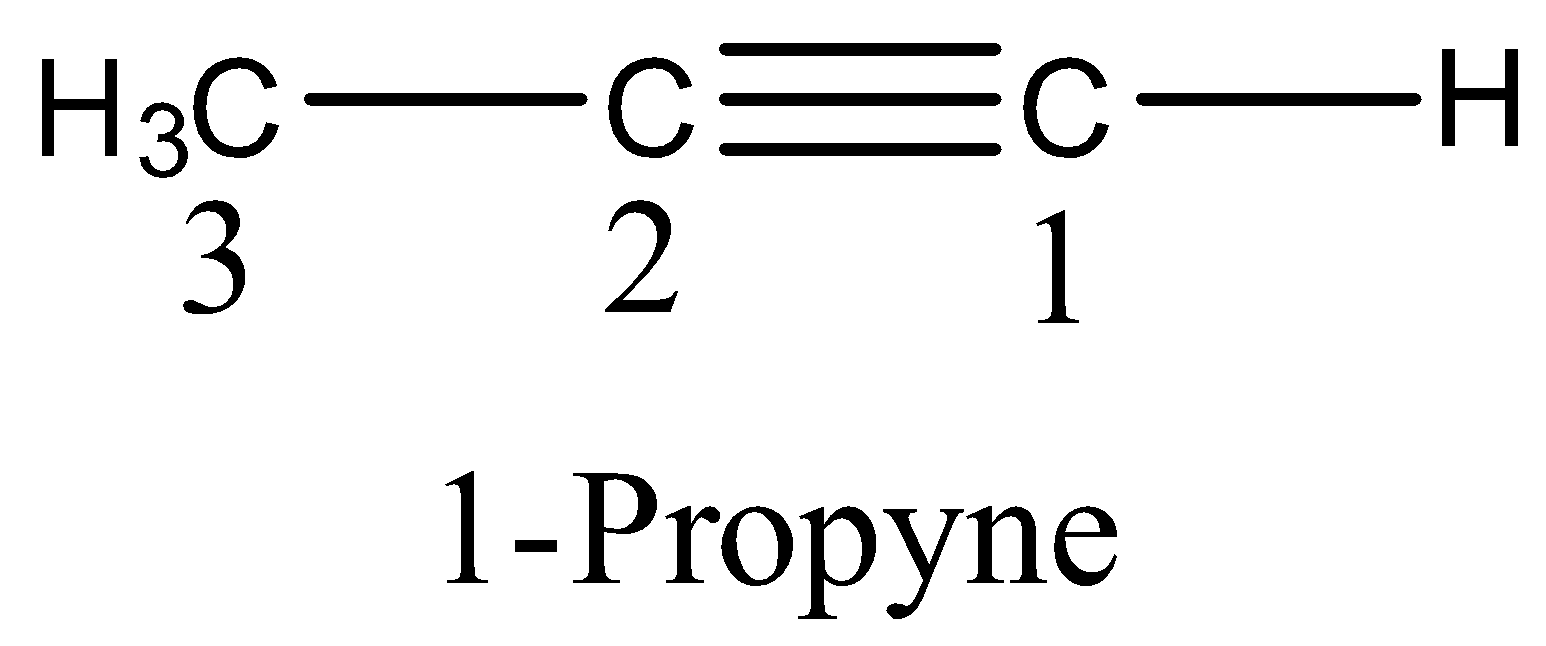
Therefore methyl acetylene is also called propyne.
So, the correct answer is “Option B”.
Additional Information:
Propyne is a colorless gas.
Propyne or methyl acetylene is the second simplest member belonging to the acetylene family.
Propyne can form explosives mixtures when reacting with air and oxidizing agents.
Propyne is used as joining torch fuel.
Note: The common names of Prop-1-yne are propyne and methyl acetylene.
The chemical reaction of methyl acetylene with oxygen is as follows.
${C{H_3}-C}\equiv {CH}+{4{O}_{2}}\to {3C{O}_{2}+{2{H}_{2}O}}$
When methyl acetylene reacts with four moles of oxygen it forms three moles of carbon dioxide and two moles of water as the product.
Acetylene contains two hydrogens, two carbons and one triple bond between two carbons. The molecular formula of acetylene is\[{{C}_{2}}{{H}_{2}}\].
Complete step by step answer:
In the question they asked the structure of the methyl acetylene.
The structure of methyl acetylene contains one more methyl group in addition to the structure of acetylene.
The structure of the methyl acetylene is as follows.
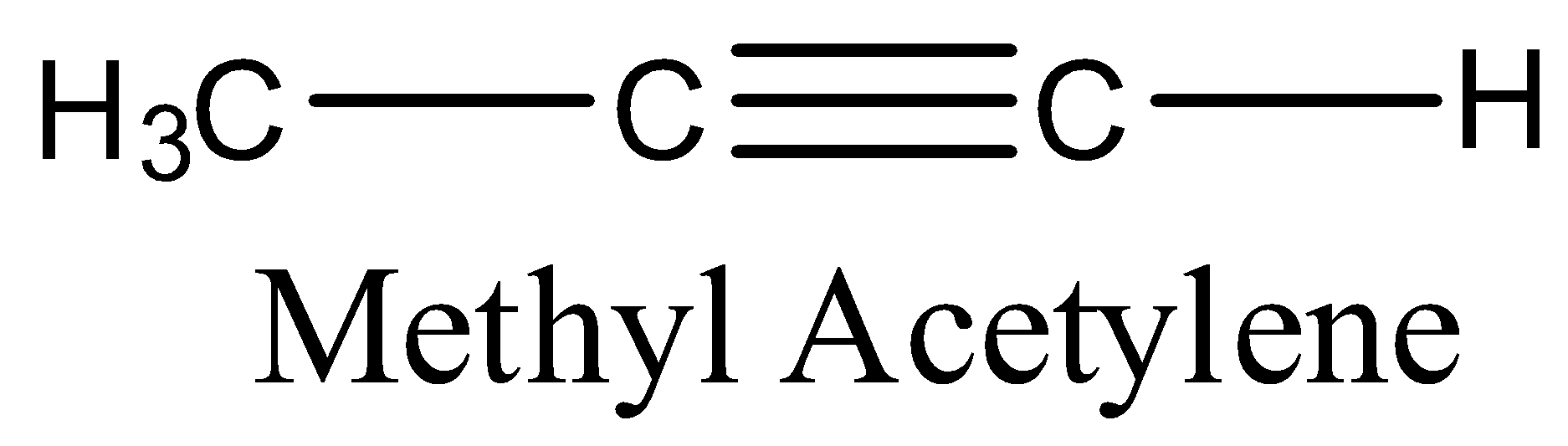
Methyl Acetylene contains four hydrogens, three carbons, one triple bond between two carbons and one single bond between two carbons.
The molecular formula of methyl acetylene is\[{{C}_{3}}{{H}_{4}}\].
Methyl acetylene is also called as propyne because it contains three carbons and one triple bond (alkyne).
Methyl acetylene is also called as Prop-1-yne (IUPAC name) because the triple is present at first carbon.
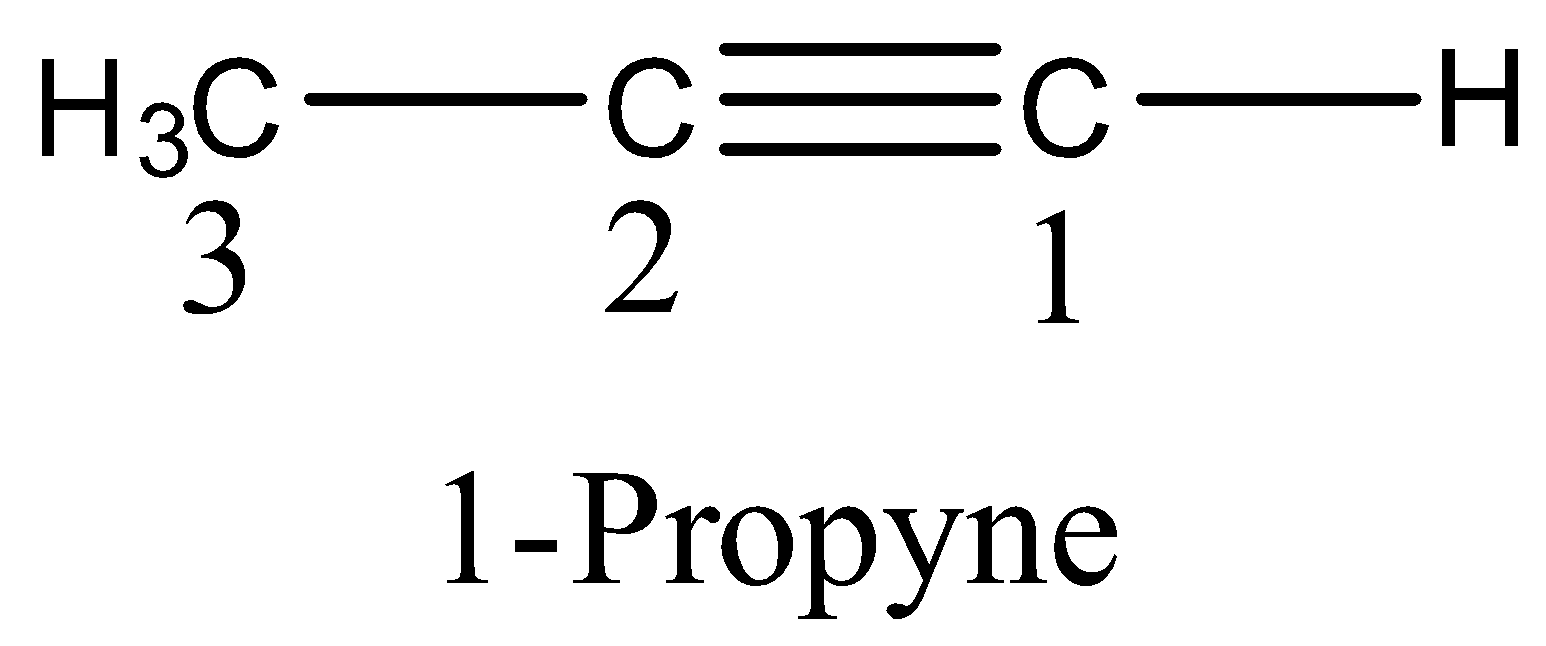
Therefore methyl acetylene is also called propyne.
So, the correct answer is “Option B”.
Additional Information:
Propyne is a colorless gas.
Propyne or methyl acetylene is the second simplest member belonging to the acetylene family.
Propyne can form explosives mixtures when reacting with air and oxidizing agents.
Propyne is used as joining torch fuel.
Note: The common names of Prop-1-yne are propyne and methyl acetylene.
The chemical reaction of methyl acetylene with oxygen is as follows.
${C{H_3}-C}\equiv {CH}+{4{O}_{2}}\to {3C{O}_{2}+{2{H}_{2}O}}$
When methyl acetylene reacts with four moles of oxygen it forms three moles of carbon dioxide and two moles of water as the product.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




