
Methane burns readily in air. Write the balanced chemical equation for it.
A. $C{H_4} + {20_2} \to C{O_2} + 2{H_2}O$
B. $C{H_4} + {30_2} \to C{O_2} + 2{H_2}O$
C. $C{H_4} + {50_2} \to 3C{O_2} + 2{H_2}O$
D.$C{H_4} + {70_2} \to 2C{O_2} + 2{H_2}O$
Answer
582k+ views
Hint: Combustion is a process in which compound burns in sufficient amounts of oxygen. Generally, carbon dioxide gas produced during combustion.
Step by step answer: Alkane is organic compound having general formula $Cn{H_2}n + 2.$
If $n = 1$ then $C{H_{2 \times 1 + 2}} = C{H_4}$ Methane. Methane is the first member of alkane.
Since, organic compounds contain carbon and hydrogen as main elements therefore they produce carbon di-oxide and water on combustion.
Methane also produces $C{O_2}$ and water on heating with oxygen or air $C{H_4}$ completely oxidized by oxygen.
$C{H_4}(g){20_{2(g)}} \to C{O_{_{2(g)}}} + 2{H_2}{O_{(1)}}$
$\Delta H = - 890KJmol{e^{ - 1}}$
Negative sign represents the exothermic reaction in which heat is evolved during reaction.
Therefore, from the above explanation the correct option is (A) $C{H_4} + {20_2} \to C{O_2} + 2{H_2}O$.
Additional Information: Methane is simplest alkane.
It is the main constituent of natural gas.
It is group$14$hydride. Methane abundantly present on earth.
Therefore, it is an economical fuel.
It is a gaseous compound so strong it processes technical challenge.
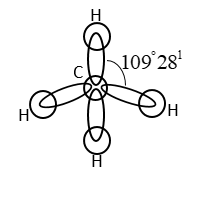
It has a tetrahedral structure. Four H-atoms are present at vertices of tetrahedron. The C-atom in methane is $s{p^3}$ hybridized.
$4s{p^3}$ hybrid orbitals of C-atoms form covalent bond with an orbital of $4$ H-atoms.
Note: Methane requires sufficient air. During incomplete combustion of alkane with insufficient amount of air carbon black is formed.
This is used in manufacture of ink, printer ink black pigments etc
Step by step answer: Alkane is organic compound having general formula $Cn{H_2}n + 2.$
If $n = 1$ then $C{H_{2 \times 1 + 2}} = C{H_4}$ Methane. Methane is the first member of alkane.
Since, organic compounds contain carbon and hydrogen as main elements therefore they produce carbon di-oxide and water on combustion.
Methane also produces $C{O_2}$ and water on heating with oxygen or air $C{H_4}$ completely oxidized by oxygen.
$C{H_4}(g){20_{2(g)}} \to C{O_{_{2(g)}}} + 2{H_2}{O_{(1)}}$
$\Delta H = - 890KJmol{e^{ - 1}}$
Negative sign represents the exothermic reaction in which heat is evolved during reaction.
Therefore, from the above explanation the correct option is (A) $C{H_4} + {20_2} \to C{O_2} + 2{H_2}O$.
Additional Information: Methane is simplest alkane.
It is the main constituent of natural gas.
It is group$14$hydride. Methane abundantly present on earth.
Therefore, it is an economical fuel.
It is a gaseous compound so strong it processes technical challenge.
It has a tetrahedral structure. Four H-atoms are present at vertices of tetrahedron. The C-atom in methane is $s{p^3}$ hybridized.
$4s{p^3}$ hybrid orbitals of C-atoms form covalent bond with an orbital of $4$ H-atoms.

Note: Methane requires sufficient air. During incomplete combustion of alkane with insufficient amount of air carbon black is formed.
This is used in manufacture of ink, printer ink black pigments etc
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




