
What is the mechanism of aromatization?
Answer
582.9k+ views
Hint:Aromatization is a reaction in which an aromatic system is formed. It can also refer to the production of new aromatic moieties in a molecule which is already aromatic. It is an aromatic system formed by a single nonaromatic precursor. It is achieved by dehydrogenation of existing cyclic compounds, illustrated by the conversion of cyclohexane into benzene.
Complete step by step answer:
An aromatic system is formed in aromatization. It is able to produce a new aromatic moiety in a molecule which is already aromatic.
Here we understand aromatization with an example and try to know the mechanism of aromatization:
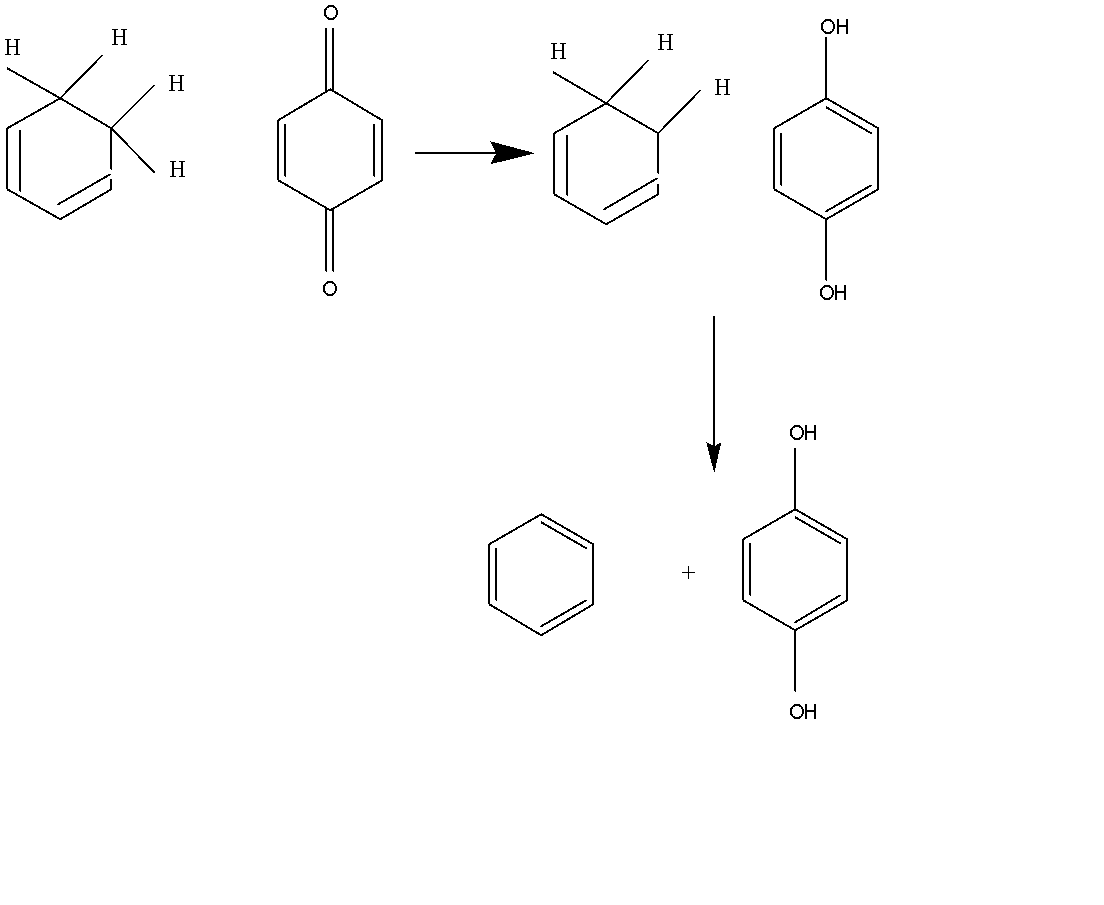
From the above mechanism the reaction starts from the aromatic compound and is given a cyclic ring of benzene.
Ethene aromatization reaction was studied at different space velocities and time on stream. The simplest naphthene is cyclohexane, Catalytic dehydrogenation converts it to benzene. Heptane is catalytically converted to toluene.
Here we understand the example of aromatization by the condensation of hydrocarbons:
$3{C_3}{H_8} \to {C_6}{H_6} + 3C{H_4} + 3{H_2}$
Propane is catalytically converted to benzene during the cracking of petroleum at $600 - {800^ \circ }C$ .
Note:Here we remember that aromatization is a reaction which is an aromatic system to form a single nonaromatic molecule. This achieved cyclic compounds, and separated by the convert in cyclohexane into benzene. There are so many examples of aromatization. This occurs in hydrocarbons also which convert cyclic rings and form benzene. There are numerous routes and means to accomplish this transformation, the simplest and most important which is direct dehydrogenation. Aromatization occurs in the human body also which converts testosterone to estrogen.
Complete step by step answer:
An aromatic system is formed in aromatization. It is able to produce a new aromatic moiety in a molecule which is already aromatic.
Here we understand aromatization with an example and try to know the mechanism of aromatization:

From the above mechanism the reaction starts from the aromatic compound and is given a cyclic ring of benzene.
Ethene aromatization reaction was studied at different space velocities and time on stream. The simplest naphthene is cyclohexane, Catalytic dehydrogenation converts it to benzene. Heptane is catalytically converted to toluene.
Here we understand the example of aromatization by the condensation of hydrocarbons:
$3{C_3}{H_8} \to {C_6}{H_6} + 3C{H_4} + 3{H_2}$
Propane is catalytically converted to benzene during the cracking of petroleum at $600 - {800^ \circ }C$ .
Note:Here we remember that aromatization is a reaction which is an aromatic system to form a single nonaromatic molecule. This achieved cyclic compounds, and separated by the convert in cyclohexane into benzene. There are so many examples of aromatization. This occurs in hydrocarbons also which convert cyclic rings and form benzene. There are numerous routes and means to accomplish this transformation, the simplest and most important which is direct dehydrogenation. Aromatization occurs in the human body also which converts testosterone to estrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




