
What is meant by isomers? Draw the structure of two isomers of butane, ${{C}_{4}}{{H}_{10}}$ . Explain why we cannot have isomers of the first three members of the alkane series?
Answer
539.7k+ views
Hint:The phenomenon is isomerism is defined as the similarity in the chemical formulas but difference in structural formulas. Due to this the isomers may show different chemical and physical properties also.
Complete step-by-step answer:Let us answer the given question in brief;
Isomers-
These are the molecules with the same molecular formula but different structural formulas. The isomers are shown by the fourth alkane in the group i.e. butane. Butane $\left( {{C}_{4}}{{H}_{10}} \right)$ shows constitutional isomers as normal butane or unbranched butane and iso-butane or i-butane (according to IUPAC the butane and 2-methyl propane respectively).
These can be shown by the below given diagrams more conveniently;
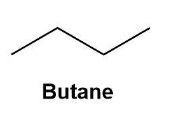
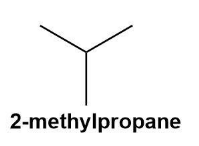
Also, the isomers can be formed due to the branching and rearrangements which is impossible in the first three alkanes.
Note:Do note that the molecule to show isomerism needs to have at least 4 carbons for the specific rearrangement. Thus, the butane is the first alkane to show the phenomenon of isomerism in nature as methane, ethane and propane cannot show the rearrangements.
Complete step-by-step answer:Let us answer the given question in brief;
Isomers-
These are the molecules with the same molecular formula but different structural formulas. The isomers are shown by the fourth alkane in the group i.e. butane. Butane $\left( {{C}_{4}}{{H}_{10}} \right)$ shows constitutional isomers as normal butane or unbranched butane and iso-butane or i-butane (according to IUPAC the butane and 2-methyl propane respectively).
These can be shown by the below given diagrams more conveniently;


Also, the isomers can be formed due to the branching and rearrangements which is impossible in the first three alkanes.
Note:Do note that the molecule to show isomerism needs to have at least 4 carbons for the specific rearrangement. Thus, the butane is the first alkane to show the phenomenon of isomerism in nature as methane, ethane and propane cannot show the rearrangements.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




