
What is meant by hybridization of atomic orbitals? Describe $ sp,s{p^2}\,and\,s{p^3} $ hybridization with examples.
Answer
504.3k+ views
Hint :Hybridization is defined as the mixing of a group of slightly different atomic orbitals, resulting in a new set of orbitals with equivalent energies and forms. One $ 2s $ orbital, for example, can combine with two $ \;2p $ orbitals of carbon to generate three new $ s{p^2} $ hybrid orbitals. Because there is less repulsion between the electron pairs in these hybrid orbitals, they are more stable. Hybridization aids in determining the molecule's geometry.
Complete Step By Step Answer:
$ sp $ Hybridization:
When one s and one p orbital in the same main shell of an atom combine to generate two new equivalent orbitals, this is known as $ sp $ hybridization. $ sp $ hybridised orbitals are the new orbitals that develop. It produces $ 180 $ -degree linear molecules.
This sort of hybridization entails combining one s and one p orbital of equal energy to create a new hybrid orbital known as a sp hybridised orbital.
Diagonal hybridization is another name for $ sp $ hybridization.
Each $ sp $ hybridised orbital has $ 50 $ percent $ s $ and $ p $ character.
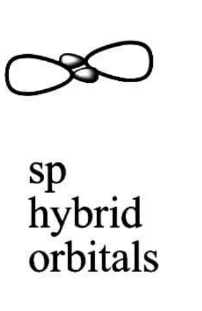
All beryllium compounds such as $ Be{F_2},{\text{ }}Be{H_2},{\text{ }}BeC{l_2} $ and all carbon-containing triple bond compounds such as $ {C_2}{H_2} $ are examples of $ sp $ Hybridization.
$ s{p^2} $ Hybridization:
When one $ s $ and two $ p $ orbitals of the same shell of an atom combine to generate three equivalent orbitals, this is known as $ s{p^2} $ hybridization. $ s{p^2} $ hybrid orbitals are the new orbitals that have been created.
Trigonal hybridization is another name for $ s{p^2} $ hybridization.
It entails combining one s orbital with two p orbitals of equal energy to create the $ s{p^2} $ hybrid orbital.
A trigonal symmetry blend of $ s $ and $ p $ orbitals is maintained at $ 1200 $ degrees.
All three hybrid orbitals remain in the same plane and form a $ 120^\circ $ angle with one another. Each of the resulting hybrid orbitals has $ 33.33 $ percent s and $ 66.66 $ percent character.
A triangle planar form is found in molecules in which the central atom is connected to three other atoms and is $ s{p^2} $ hybridised.
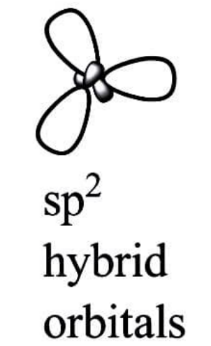
Examples:
$ B{F_3},{\text{ }}B{H_3}, $ and all other Boron compounds
Ethylene is a group of carbon molecules that have a carbon-carbon double bond $ \left( {{C_2}{H_4}} \right) $
$ s{p^3} $ Hybridization:
A tetrahedral hybridization, or $ s{p^3}, $ occurs when one orbital and three orbitals belonging to the same shell of an atom combine to generate four new equivalent orbitals. $ s{p^3} $ hybrid orbitals are the new orbitals that have been generated.
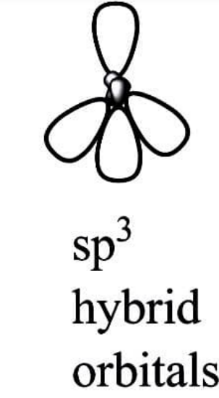
These are aimed at the four corners of a conventional tetrahedron and form a $ 109^\circ 28' $ angle with each other.
The $ s{p^3} $ hybrid orbitals have a $ 109.280 $ degree angle between them.
Each $ s{p^3} $ hybrid orbital contains $ 25\% $ s character and $ 75\% $ p character.
Ethane $ \left( {{C_2}{H_6}} \right) $ and methane are two examples of $ s{p^3} $ hybridization.
Note :
The hybrid orbitals have a different geometry of orbital arrangement and energy than the conventional atomic orbitals after hybridization. In addition, the orbital overlap reduces the molecule's energy. Degenerate hybrid orbitals are generated by combining conventional atomic orbitals with degenerate hybrid orbitals.
Complete Step By Step Answer:
$ sp $ Hybridization:
When one s and one p orbital in the same main shell of an atom combine to generate two new equivalent orbitals, this is known as $ sp $ hybridization. $ sp $ hybridised orbitals are the new orbitals that develop. It produces $ 180 $ -degree linear molecules.
This sort of hybridization entails combining one s and one p orbital of equal energy to create a new hybrid orbital known as a sp hybridised orbital.
Diagonal hybridization is another name for $ sp $ hybridization.
Each $ sp $ hybridised orbital has $ 50 $ percent $ s $ and $ p $ character.

All beryllium compounds such as $ Be{F_2},{\text{ }}Be{H_2},{\text{ }}BeC{l_2} $ and all carbon-containing triple bond compounds such as $ {C_2}{H_2} $ are examples of $ sp $ Hybridization.
$ s{p^2} $ Hybridization:
When one $ s $ and two $ p $ orbitals of the same shell of an atom combine to generate three equivalent orbitals, this is known as $ s{p^2} $ hybridization. $ s{p^2} $ hybrid orbitals are the new orbitals that have been created.
Trigonal hybridization is another name for $ s{p^2} $ hybridization.
It entails combining one s orbital with two p orbitals of equal energy to create the $ s{p^2} $ hybrid orbital.
A trigonal symmetry blend of $ s $ and $ p $ orbitals is maintained at $ 1200 $ degrees.
All three hybrid orbitals remain in the same plane and form a $ 120^\circ $ angle with one another. Each of the resulting hybrid orbitals has $ 33.33 $ percent s and $ 66.66 $ percent character.
A triangle planar form is found in molecules in which the central atom is connected to three other atoms and is $ s{p^2} $ hybridised.

Examples:
$ B{F_3},{\text{ }}B{H_3}, $ and all other Boron compounds
Ethylene is a group of carbon molecules that have a carbon-carbon double bond $ \left( {{C_2}{H_4}} \right) $
$ s{p^3} $ Hybridization:
A tetrahedral hybridization, or $ s{p^3}, $ occurs when one orbital and three orbitals belonging to the same shell of an atom combine to generate four new equivalent orbitals. $ s{p^3} $ hybrid orbitals are the new orbitals that have been generated.

These are aimed at the four corners of a conventional tetrahedron and form a $ 109^\circ 28' $ angle with each other.
The $ s{p^3} $ hybrid orbitals have a $ 109.280 $ degree angle between them.
Each $ s{p^3} $ hybrid orbital contains $ 25\% $ s character and $ 75\% $ p character.
Ethane $ \left( {{C_2}{H_6}} \right) $ and methane are two examples of $ s{p^3} $ hybridization.
Note :
The hybrid orbitals have a different geometry of orbital arrangement and energy than the conventional atomic orbitals after hybridization. In addition, the orbital overlap reduces the molecule's energy. Degenerate hybrid orbitals are generated by combining conventional atomic orbitals with degenerate hybrid orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




