
When m-bromonitrobenzene is heated with alcoholic KCN at $200^\circ {\rm{C}}$
and then hydrolysed, the product formed is:
This question has multiple correct options
A.
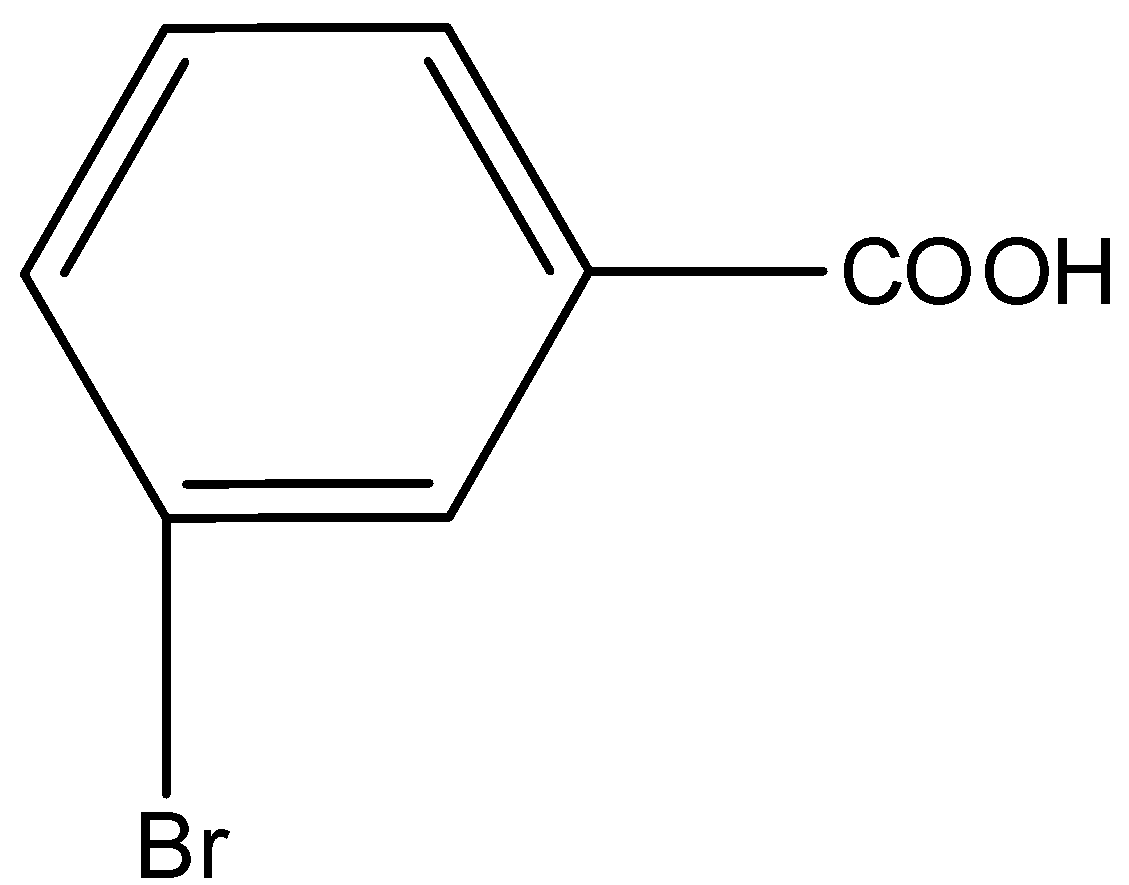
B.
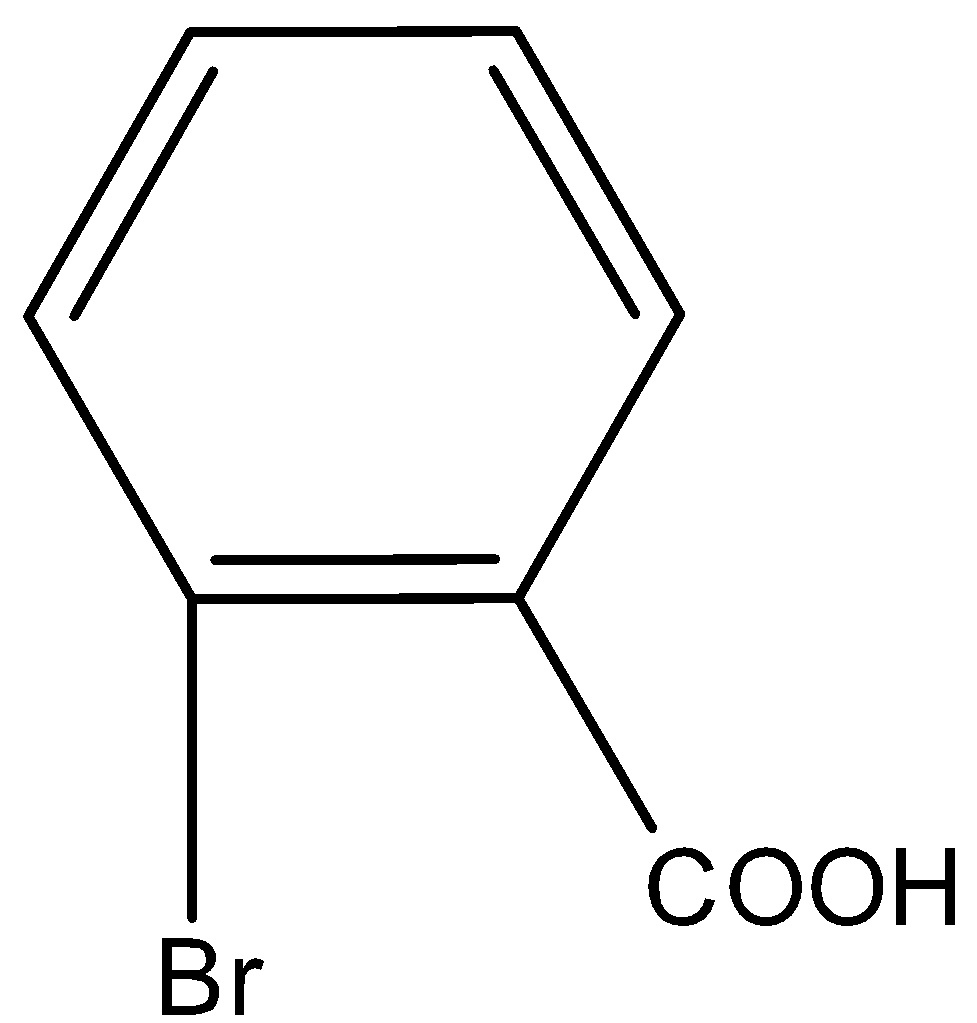
C.
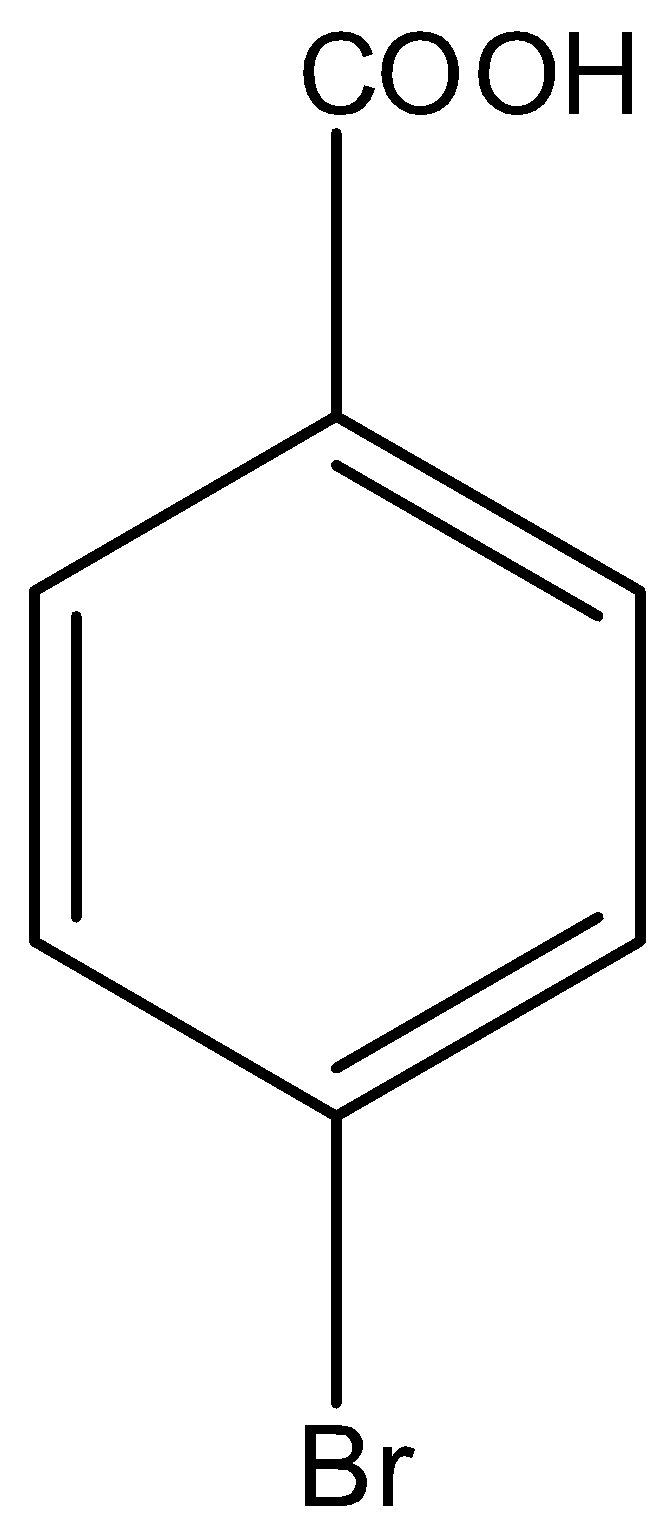
D.
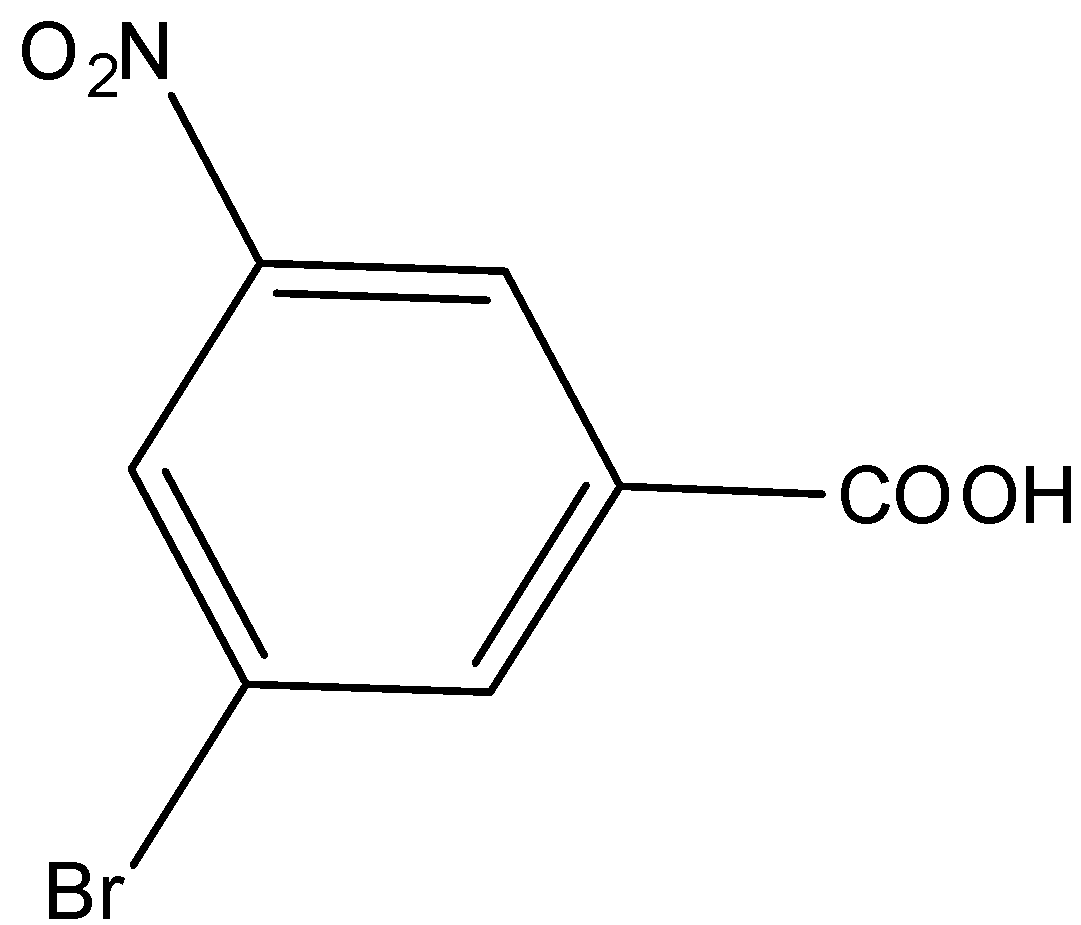




Answer
572.1k+ views
Hint: We know that aromatic nitro compounds are those compounds in which a nitro group $\left( {{\rm{N}}{{\rm{O}}_{\rm{2}}}} \right)$is bonded to a benzene ring. Nitro group is an electron withdrawing group that deactivates the aromatic ring when bonded to a benzene ring.
Complete step by step answer:
Let’s discuss a reaction whose name is Von-Richter reaction. In this reaction, an aromatic nitro compound undergoes reaction with KCN (potassium cyanide) in aqueous ethanol to result in the product of sine substitution by –COOH group. The sine substitution is the ring substitution in which substitution occurs at the adjacent position of the leaving group.
Here, we have to identify the product of heating of m-bromonitrobenzene with alcoholic KCN at $200^\circ {\rm{C}}$ and hydrolysed. m-bromonitrobenzene undergoes Von-Richter reaction to give the mixture of ortho and para mixture of bromobenzoic acid. The reaction is shown as below.
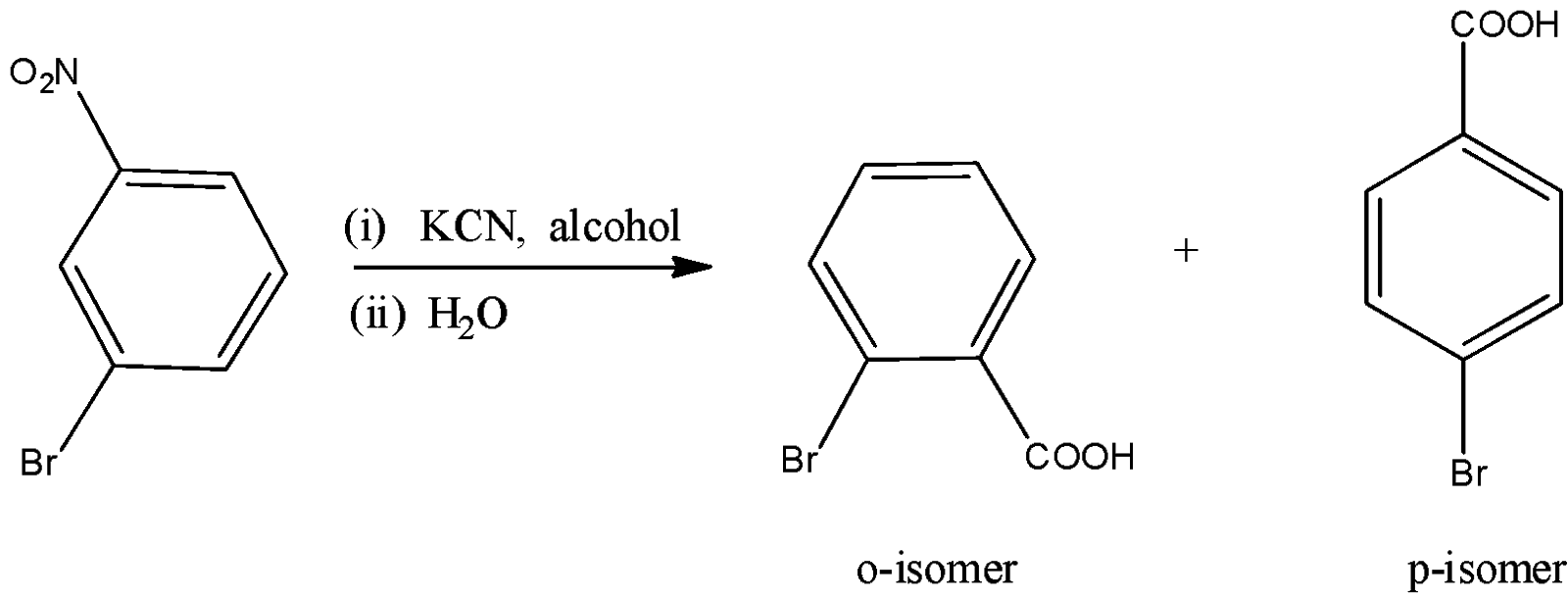
So, the correct answer is Option B,C.
Additional Information:
Let’s understand the three isomers of benzene derivatives. The three isomers are o-isomer, p-isomer and m-isomer.
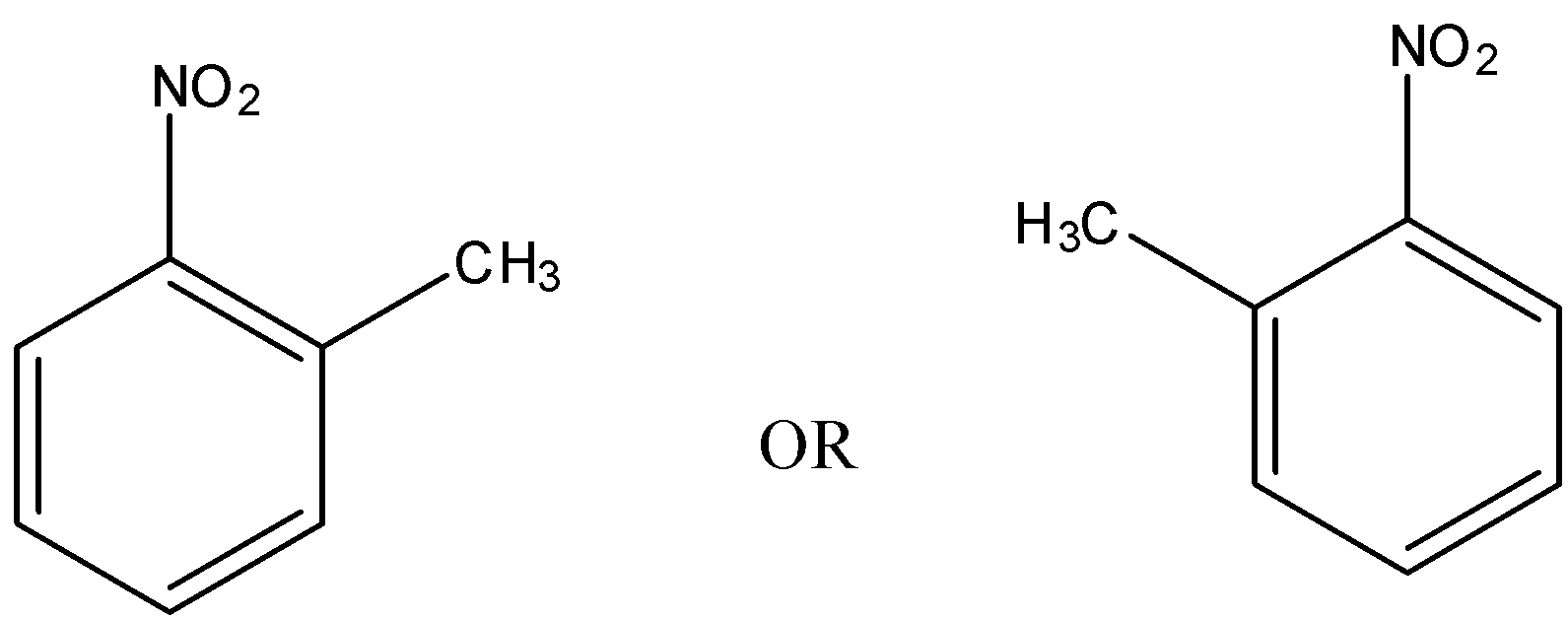
o-isomer is the isomer in which a substituent is bonded to the adjacent carbon atom to which a functional group is attached.
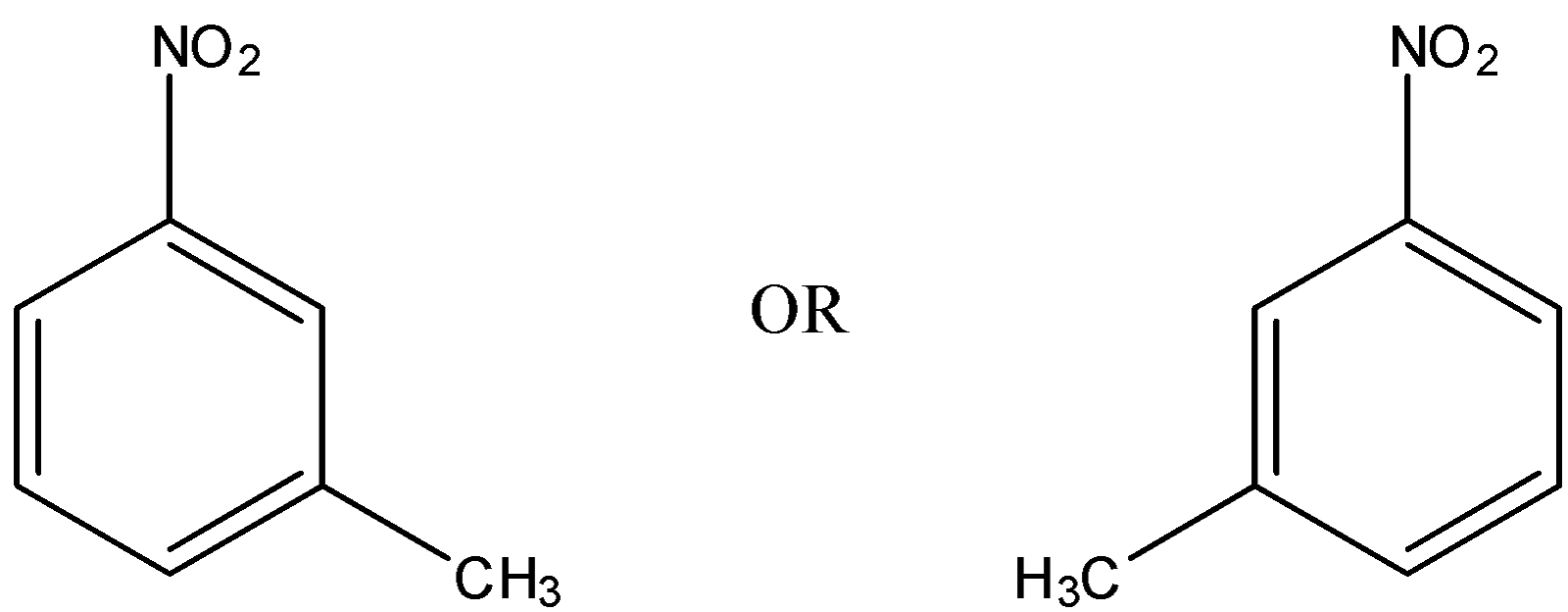
The m-isomer is the isomer in which a substituent is bonded to the carbon atom that is in 2 position to the carbon atom to which a functional group is attached.
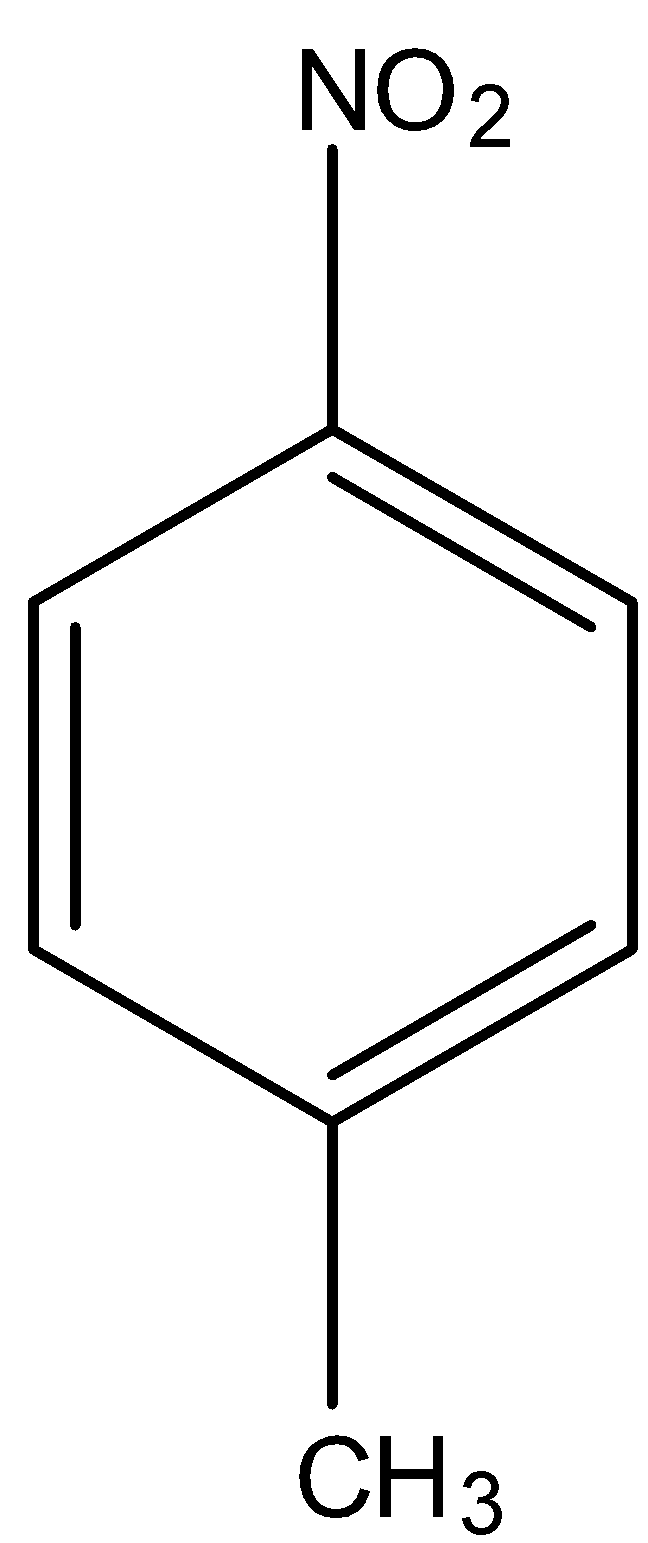
The p-isomer is the isomer in which a substituent is bonded to the carbon atom that is in 3 position to the carbon atom to which a functional group is attached.
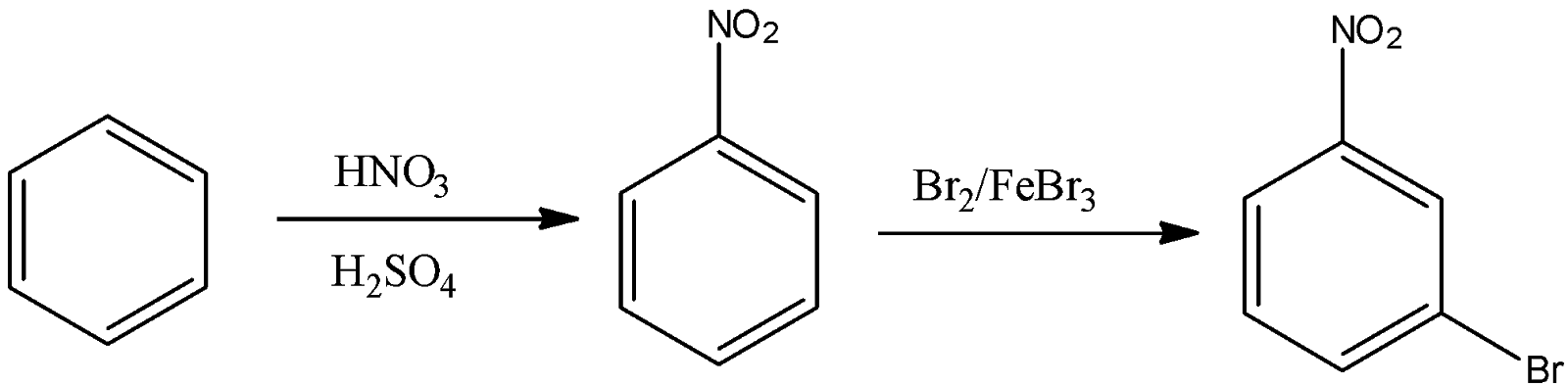
Note: The preparation of m-bromonitrobenzene is a two step process. First, we have to react with benzene with nitric acid in presence of sulphuric acid, so that nitration of benzene takes place. Then the nitrobenzene is to be reacted with ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ in presence of ${\rm{FeB}}{{\rm{r}}_{\rm{3}}}$to form m-bromonitrobenzene.
Complete step by step answer:
Let’s discuss a reaction whose name is Von-Richter reaction. In this reaction, an aromatic nitro compound undergoes reaction with KCN (potassium cyanide) in aqueous ethanol to result in the product of sine substitution by –COOH group. The sine substitution is the ring substitution in which substitution occurs at the adjacent position of the leaving group.
Here, we have to identify the product of heating of m-bromonitrobenzene with alcoholic KCN at $200^\circ {\rm{C}}$ and hydrolysed. m-bromonitrobenzene undergoes Von-Richter reaction to give the mixture of ortho and para mixture of bromobenzoic acid. The reaction is shown as below.

So, the correct answer is Option B,C.
Additional Information:
Let’s understand the three isomers of benzene derivatives. The three isomers are o-isomer, p-isomer and m-isomer.
o-isomer is the isomer in which a substituent is bonded to the adjacent carbon atom to which a functional group is attached.

The m-isomer is the isomer in which a substituent is bonded to the carbon atom that is in 2 position to the carbon atom to which a functional group is attached.

The p-isomer is the isomer in which a substituent is bonded to the carbon atom that is in 3 position to the carbon atom to which a functional group is attached.

Note: The preparation of m-bromonitrobenzene is a two step process. First, we have to react with benzene with nitric acid in presence of sulphuric acid, so that nitration of benzene takes place. Then the nitrobenzene is to be reacted with ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ in presence of ${\rm{FeB}}{{\rm{r}}_{\rm{3}}}$to form m-bromonitrobenzene.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




