
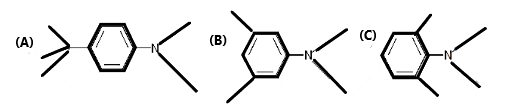
Maximum effect of steric inhibition of resonance can be expected in:

Answer
547.5k+ views
Hint: Resonance is referred to as the phenomenon in which the pi-electrons or the electrons in the p-orbitals are delocalized in the entire molecule resulting in the formation of a stable structure that has equal concentration of electrons both above and below the plane of the molecule undergoing resonance.
Complete stepwise Solution
In the question there are three compounds in which the common substituent is the N, N-dimethyl amine group.
In figure A, there is a tert-butyl group at the para position of the molecule which is an electron releasing group and being present in the para position it favours “extended delocalization of electrons.”
In the figure B, there are two methyl groups at the meta positions of the molecule which do not inhibit resonance but do not contribute to it as well.
In the figure C, there are two methyl groups at the ortho-positions which, due to their bulkiness, repulses the electron cloud of the methyl groups of N, N-dimethyl amine, and therefore forces it to be out of plane and hence inhibits resonance.
Hence, Maximum effect of steric inhibition of resonance can be expected in Figure C and the least is to be expected from figure A.
Note
As resonance involves the delocalization of the pi-electrons or the p-electrons on the entire molecule, for the successful delocalization of the pi-electron cloud, the structure needs to be planned first of all. Secondly, there are different substituents that can be attached to the carbon atoms that have undergone resonance. Among these substituents, those that have electron releasing effect are in the favour of resonance. Thirdly, the position of the substituents is also a factor that governs the resonance in the molecule. If the substituents are placed in such positions which inhibit the resonance then it is called the Steric Inhibition of Resonance or (SIR).
Complete stepwise Solution
In the question there are three compounds in which the common substituent is the N, N-dimethyl amine group.
In figure A, there is a tert-butyl group at the para position of the molecule which is an electron releasing group and being present in the para position it favours “extended delocalization of electrons.”
In the figure B, there are two methyl groups at the meta positions of the molecule which do not inhibit resonance but do not contribute to it as well.
In the figure C, there are two methyl groups at the ortho-positions which, due to their bulkiness, repulses the electron cloud of the methyl groups of N, N-dimethyl amine, and therefore forces it to be out of plane and hence inhibits resonance.
Hence, Maximum effect of steric inhibition of resonance can be expected in Figure C and the least is to be expected from figure A.
Note
As resonance involves the delocalization of the pi-electrons or the p-electrons on the entire molecule, for the successful delocalization of the pi-electron cloud, the structure needs to be planned first of all. Secondly, there are different substituents that can be attached to the carbon atoms that have undergone resonance. Among these substituents, those that have electron releasing effect are in the favour of resonance. Thirdly, the position of the substituents is also a factor that governs the resonance in the molecule. If the substituents are placed in such positions which inhibit the resonance then it is called the Steric Inhibition of Resonance or (SIR).
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




