
Maximum content of iron is present in
A. Ferritin
B. Myoglobin
C. Haemoglobin
D. Cytochrome
Answer
584.1k+ views
Hint: Ferritin is an iron storing protein, myoglobin is an oxygen-carrying protein, haemoglobin is a chromoprotein while cytochrome is a plant protein.
Step by step answer:Ferritin – The ferritin is a protein that is used to store iron inside the cells. Hence, it is a blood cell protein that contains iron. The amount of iron in ferritin fluctuates according to the storage available in the red blood cells. Each ferritin complex can store about 4500 iron ions through 3 fold polar channels.
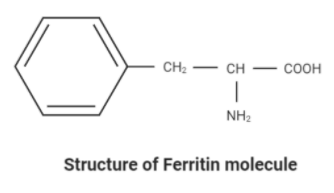
In the structure, the molecule has no iron ion. Therefore, Ferritin has 0 iron content in it.
Myoglobin – Myoglobin is an oxygen-carrying protein in our muscles. It is found in the skeletal muscle tissue. Myoglobin is distantly related to hemoglobin in its structure.
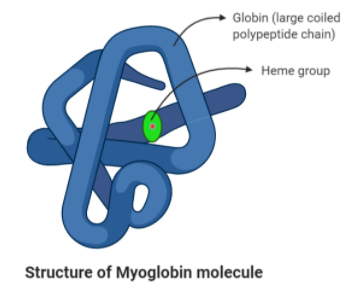
In the structure, the molecule has one heme group. The heme group consists of an atom of ferrous iron that is surrounded by the porphyrin ring. The porphyrin ring is nothing but four nitrogen-containing pyrrole molecules. Hence, we can say that the myoglobin molecule has 1 Fe ion.
Haemoglobin – Haemoglobin is a pigment that is present in the red blood cells of the blood. It is a chromoprotein as it gives the red color to the blood because of the Heme cofactor. It helps in the process of respiration. The structure of the Hemoglobin consists of four polypeptide chains with a heme group each, just like myoglobin coiled together. Hence, it is an oligoprotein.
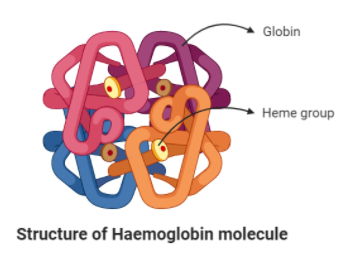
In the structure, the molecule has a quaternary chain. This means that each of the four polypeptide chains has a heme group with iron in the center. The four chains consist of two alpha and two beta chains. These chains are linked together by the disulfide bonds and stabilized by the hydrogen bonds. Each ferrous ion combines with an atom of oxygen.
Cytochrome – The cytochrome is a plant protein. They help in the Electron Transport Chain by playing an important role in the generation of ATP. They help in the transfer of the electrons by the process of oxidation and reduction during the ETS. The molecule of cytochrome has one heme group.
Thus, the increasing content of the iron in the various molecules is as follows –
Ferritin < Myoglobin = Cytochrome < Hemoglobin.
Therefore, Hemoglobin has the highest iron content.
Hence, option (C) is correct
Note: A ferritin molecule has 0 iron in it. It just stores iron in itself, but in actual, it carries no iron. Cytochromes are iron-containing proteins. It helps in the generation of ATP by an electron transport chain. It helps in the transfer of electrons by oxidation and reduction. It has one heme group.
Step by step answer:Ferritin – The ferritin is a protein that is used to store iron inside the cells. Hence, it is a blood cell protein that contains iron. The amount of iron in ferritin fluctuates according to the storage available in the red blood cells. Each ferritin complex can store about 4500 iron ions through 3 fold polar channels.

In the structure, the molecule has no iron ion. Therefore, Ferritin has 0 iron content in it.
Myoglobin – Myoglobin is an oxygen-carrying protein in our muscles. It is found in the skeletal muscle tissue. Myoglobin is distantly related to hemoglobin in its structure.

In the structure, the molecule has one heme group. The heme group consists of an atom of ferrous iron that is surrounded by the porphyrin ring. The porphyrin ring is nothing but four nitrogen-containing pyrrole molecules. Hence, we can say that the myoglobin molecule has 1 Fe ion.
Haemoglobin – Haemoglobin is a pigment that is present in the red blood cells of the blood. It is a chromoprotein as it gives the red color to the blood because of the Heme cofactor. It helps in the process of respiration. The structure of the Hemoglobin consists of four polypeptide chains with a heme group each, just like myoglobin coiled together. Hence, it is an oligoprotein.

In the structure, the molecule has a quaternary chain. This means that each of the four polypeptide chains has a heme group with iron in the center. The four chains consist of two alpha and two beta chains. These chains are linked together by the disulfide bonds and stabilized by the hydrogen bonds. Each ferrous ion combines with an atom of oxygen.
Cytochrome – The cytochrome is a plant protein. They help in the Electron Transport Chain by playing an important role in the generation of ATP. They help in the transfer of the electrons by the process of oxidation and reduction during the ETS. The molecule of cytochrome has one heme group.
Thus, the increasing content of the iron in the various molecules is as follows –
Ferritin < Myoglobin = Cytochrome < Hemoglobin.
Therefore, Hemoglobin has the highest iron content.
Hence, option (C) is correct
Note: A ferritin molecule has 0 iron in it. It just stores iron in itself, but in actual, it carries no iron. Cytochromes are iron-containing proteins. It helps in the generation of ATP by an electron transport chain. It helps in the transfer of electrons by oxidation and reduction. It has one heme group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




