
Match the following.
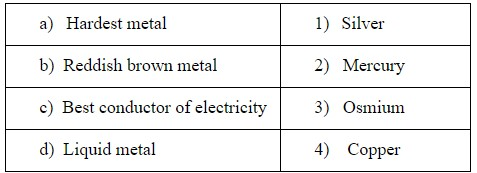
1.a—4, b—3, c—2, d—1
2.a—4, b—2, c—3, d—4
3.a—2, b—1, c—4, d—3
4.a—3, b—4, c—1, d—2

Answer
603.6k+ views
Hint: Have you ever tried to fix a faulty connection at home? You must have seen a thin reddish brown wire underneath the plastic wrapping. Have you ever wondered, what that wire is made up of?
If you will recall, there are only two metals which exist in liquid form at room temperature. And the one which we have in our question is also used in thermometers.
Complete step by step solution:
Hardness is the ability of a metal to resist deformation; it is determined by a standard test where resistance of metal to indentation is measured. Among the following Osmium is the hardest metal as it belongs to the platinum group metals and possesses a high density. It is the densest naturally occurring element with melting point 3033℃.
Silver and copper are coinage metals. So, they can be easily molded into different shapes while mercury is liquid at room temperature.
The reason why any object is colored is that they absorb some wavelengths of light and reflect other wavelengths of light. When light is shined upon copper metal, its atoms absorb some of the light in the blue-green region of the spectrum When a metal absorbs one color of light, its complementary color is reflected back to our eyes. Since copper absorbs the light of the blue green region, its complementary color is red-orange which is reflected. Hence copper appears a reddish-brown in color.
In metals electrical conductivity is the result of movement of electrically charged particles. The free electrons in the metal allows them to conduct electricity. Silver has the freest electrons followed by copper. So, the best conductor of electricity is silver.
Liquidity is the property of the substance to flow but it has a constant volume. The only metal that is liquid at normal temperature and pressure because it is bad at sharing electrons. The electrons in mercury atoms are bound more tightly to the nucleus. Only a little heat is required to overcome the weak binding between mercury atoms. Because of the behavior of the valence electrons, mercury has a low melting point. Therefore, mercury is the liquid metal among the following.
So, from the above points we can conclude that the correct option (4).
Note: Even though silver is the best conductor of electricity, generally copper is used in electrical appliances because copper is highly conductive metal and allows for a greater distance of electrical current travel.
If you will recall, there are only two metals which exist in liquid form at room temperature. And the one which we have in our question is also used in thermometers.
Complete step by step solution:
Hardness is the ability of a metal to resist deformation; it is determined by a standard test where resistance of metal to indentation is measured. Among the following Osmium is the hardest metal as it belongs to the platinum group metals and possesses a high density. It is the densest naturally occurring element with melting point 3033℃.
Silver and copper are coinage metals. So, they can be easily molded into different shapes while mercury is liquid at room temperature.
The reason why any object is colored is that they absorb some wavelengths of light and reflect other wavelengths of light. When light is shined upon copper metal, its atoms absorb some of the light in the blue-green region of the spectrum When a metal absorbs one color of light, its complementary color is reflected back to our eyes. Since copper absorbs the light of the blue green region, its complementary color is red-orange which is reflected. Hence copper appears a reddish-brown in color.
In metals electrical conductivity is the result of movement of electrically charged particles. The free electrons in the metal allows them to conduct electricity. Silver has the freest electrons followed by copper. So, the best conductor of electricity is silver.
Liquidity is the property of the substance to flow but it has a constant volume. The only metal that is liquid at normal temperature and pressure because it is bad at sharing electrons. The electrons in mercury atoms are bound more tightly to the nucleus. Only a little heat is required to overcome the weak binding between mercury atoms. Because of the behavior of the valence electrons, mercury has a low melting point. Therefore, mercury is the liquid metal among the following.
So, from the above points we can conclude that the correct option (4).
Note: Even though silver is the best conductor of electricity, generally copper is used in electrical appliances because copper is highly conductive metal and allows for a greater distance of electrical current travel.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




