
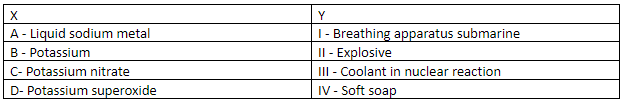
Match the compounds/metal in X with their use in Y. Hence correct order is:
i. A-I,B-III,C-II,D-IV
ii. A-III,B-IV,C-II,D-I
iii. A-II,B-I,C-III,D-IV
iv. A-IV,B-II,C-III,D-I

Answer
539.1k+ views
Hint: Earlier water was used as a coolant in the reactors now for high pressure reactor molten metals are used. Alkali metals are used as soap in the form of hydroxides. Superoxides occur as a source for oxygen.
Complete answer:
The boiling point of water is much lower than that of molten metals. For very high power nuclear reactors instead of pressurised water we use molten metal for deep cooling. Various metals are used in the nuclear reactors. The metal coolants are more effective in removing heat more rapidly. Sodium in liquid form is widely used as a coolant because it does not corrode steel and other alloys that are used in reactors. Hence, option A matches with III.
Potassium is widely used in the formation of soap. Both potassium and sodium are suitable for soap formation but the soaps of potassium are more soluble than that of the sodium salt. Potassium soaps are known as soft soaps when present in concentrated forms. Option B matches with IV.
Potassium nitrate is used in gunpowder. This creates an explosion under fire or pressure conditions. When we heat potassium nitrate it forms potassium nitrite and oxygen. Option C matches with II.
As the amount of oxygen is less under water. The use of superoxide of potassium occurs as a source of oxygen as chemical oxygen generators. Option D matches with I.
Hence, the correct option is B.
Note: Potassium nitrate is also used as a rocket fuel. Potassium nitrate is an ionic salt and occurs naturally as a mineral. The other uses include its use in fertilizers and fireworks.
Complete answer:
The boiling point of water is much lower than that of molten metals. For very high power nuclear reactors instead of pressurised water we use molten metal for deep cooling. Various metals are used in the nuclear reactors. The metal coolants are more effective in removing heat more rapidly. Sodium in liquid form is widely used as a coolant because it does not corrode steel and other alloys that are used in reactors. Hence, option A matches with III.
Potassium is widely used in the formation of soap. Both potassium and sodium are suitable for soap formation but the soaps of potassium are more soluble than that of the sodium salt. Potassium soaps are known as soft soaps when present in concentrated forms. Option B matches with IV.
Potassium nitrate is used in gunpowder. This creates an explosion under fire or pressure conditions. When we heat potassium nitrate it forms potassium nitrite and oxygen. Option C matches with II.
As the amount of oxygen is less under water. The use of superoxide of potassium occurs as a source of oxygen as chemical oxygen generators. Option D matches with I.
Hence, the correct option is B.
Note: Potassium nitrate is also used as a rocket fuel. Potassium nitrate is an ionic salt and occurs naturally as a mineral. The other uses include its use in fertilizers and fireworks.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




